FIGURE 5.
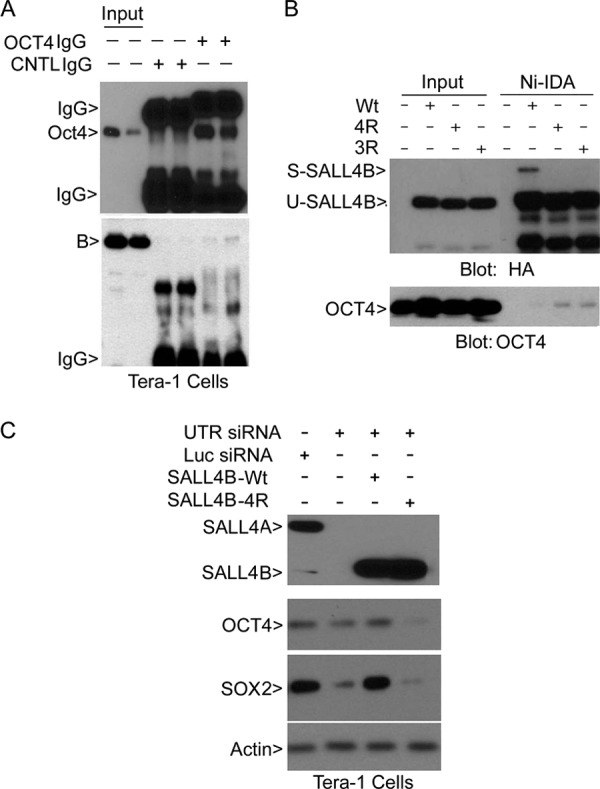
SALL4B sumoylation regulates its physical interaction with OCT4, as well as expression of Oct4 and SOX2. A, Tera-1 cell lysates in duplicate were immunoprecipitated with OCT4 IgG or control IgG. OCT4 immunoprecipitates, along with the lysate inputs, were blotted with antibodies to OCT4 and SALL4. B, HEK293T cells were transfected with wild-type SALL4B or its mutants for 48 h. 3R and 4R stand for SALL4B mutants with three (K156, K316, and K401) and four (K156, K316, K374, and K401) lysine residues replaces with arginines, respectively. Ectopically expressed SALL4B or its mutant proteins were enriched by incubation with Ni-IDA resin. Proteins bound to the resin, along with the lysate input, were blotted with antibodies to HA and OCT4. Tera-1 cells were co-transfected with SALL4 siRNA (or control siRNA) and SALL4B (or SALL4B-4R mutant) for 48 h, after which equal amounts of cell lysates were blotted with antibodies to SALL4 and HA. C, Tera-1 cells were co-transfected with SALL4 siRNA (or luciferase siRNA) and a plasmid expressing wild-type, or sumoylation-resistant, SALL4B for 48 h. Cells were then lysed, and equal amounts of cell lysates were blotted with antibodies to SALL4, OCT4, SOX2, and β-actin.
