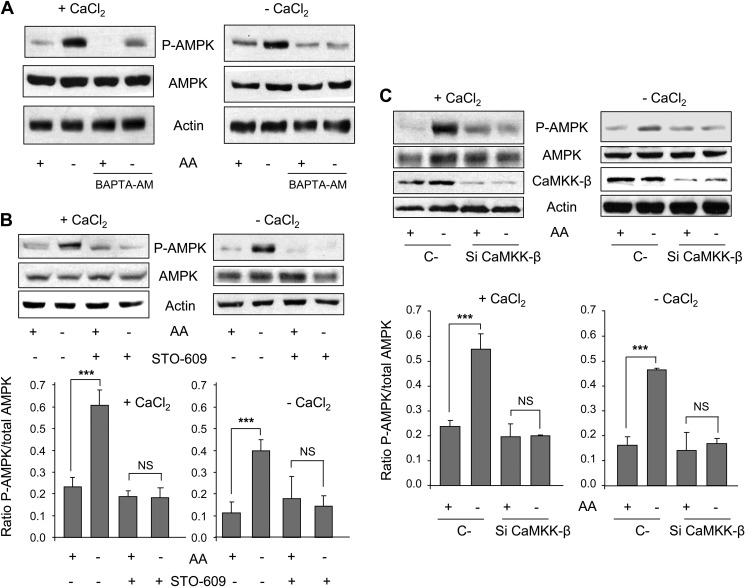
FIGURE 2.
Withdrawal of amino acids activates AMPK phosphorylation in a CaMKK-β-dependent way. A, NIH3T3 cells were grown for 2 h in KH and AA with (+CaCl2) or without CaCl2 and supplemented with 100 μm EGTA (−CaCl2) in the presence or absence of 10 μm BAPTA-AM. In the last 30 min of incubation, AA were removed or not, as indicated. Cell extracts (75 μg of protein) were analyzed by immunoblot using anti-AMPK, anti-P-AMPK (Thr-172) and, as a loading control, anti-actin antibodies. B, NIH3T3 cells were grown for 60 min in KH and AA with (+CaCl2) or without CaCl2 and supplemented with 100 μm EGTA (−CaCl2) in the presence or absence of 25 μm STO-609. In the last 30 min of incubation, AA were removed or not as indicated. Cell extracts (75 μg of protein) were analyzed by immunoblot using the same antibodies as in A. NS, not significant. C, NIH3T3 cells were transfected with CaMKK-β (Si CaMKK-β) or with negative control (C-) siRNAs. After 72 h, cells were incubated as above and analyzed by immunoblot using the same antibodies as in A plus anti-CaMKK-β. Densitometric measurements of the ratio of P-AMPK to total AMPK from four independent experiments in each case are shown below in B and C. Differences were found to be statistically significant at p < 0.0005 (***). NS, no significant difference.