Background: Rhabdomyosarcomas (RMS) are often resistant to TRAIL-induced apoptosis.
Results: IAP inhibitors prime RMS cells to TRAIL receptor 2-induced apoptosis in a RIP1-dependent but TNFα-independent manner.
Conclusion: RIP1 is a critical regulator of IAP inhibitor/TRAIL receptor 2-triggered apoptosis.
Significance: These findings have important implications for the development of novel treatment strategies for RMS.
Keywords: Apoptosis, Cancer, Rhabdomyosarcoma, RIP, Trail, IAP Proteins
Abstract
Searching for new strategies to trigger apoptosis in rhabdomyosarcoma (RMS), we investigated the effect of two novel classes of apoptosis-targeting agents, i.e. monoclonal antibodies against TNF-related apoptosis-inducing ligand (TRAIL) receptor 1 (mapatumumab) and TRAIL receptor 2 (lexatumumab) and small-molecule inhibitors of inhibitor of apoptosis (IAP) proteins. Here, we report that IAP inhibitors synergized with lexatumumab, but not with mapatumumab, to reduce cell viability and to induce apoptosis in several RMS cell lines in a highly synergistic manner (combination index <0.1). Cotreatment-induced apoptosis was accompanied by enhanced activation of caspase-8, -9, and -3; loss of mitochondrial membrane potential; and caspase-dependent apoptosis. In addition, IAP inhibitor and lexatumumab cooperated to stimulate the assembly of a cytosolic complex containing RIP1, FADD, and caspase-8. Importantly, knockdown of RIP1 by RNA interference prevented the formation of the RIP1·FADD·caspase-8 complex and inhibited subsequent activation of caspase-8, -9, and -3; loss of mitochondrial membrane potential; and apoptosis upon treatment with IAP inhibitor and lexatumumab. In addition, RIP1 silencing rescued clonogenic survival of cells treated with the combination of lexatumumab and IAP inhibitor, thus underscoring the critical role of RIP1 in cotreatment-induced apoptosis. By comparison, the TNFα-blocking antibody Enbrel had no effect on IAP inhibitor/lexatumumab-induced apoptosis, indicating that an autocrine TNFα loop is dispensable. By demonstrating that IAP inhibitors and lexatumumab synergistically trigger apoptosis in a RIP1-dependent but TNFα-independent manner in RMS cells, our findings substantially advance our understanding of IAP inhibitor-mediated regulation of TRAIL-induced cell death.
Introduction
TNF-related apoptosis-inducing ligand (TRAIL)2 directly engages the extrinsic (death receptor) pathway of apoptosis and is therefore considered as a promising cancer therapeutic (1). Upon binding of TRAIL to its receptors, a death-inducing signaling complex is assembled that drives caspase-8 activation (1). In turn, activated caspase-8 either cleaves downstream effector caspases such as caspase-3 or engages the intrinsic (mitochondrial) pathway of apoptosis. Mitochondrial outer membrane permeabilization involves the release of cytochrome c and second mitochondria-derived activator of caspases (Smac) into the cytosol, where cytochrome c promotes caspase-9 activation, whereas Smac antagonizes inhibitor of apoptosis (IAP) proteins (2).
Resistance to TRAIL-induced apoptosis frequently occurs in human cancers, e.g. due to the dominance of anti-apoptotic programs (3). For example, many cancers harbor high levels of IAP proteins (4). In an attempt to antagonize IAP proteins, small-molecule IAP inhibitors were designed that mimic the N-terminal part of Smac and interfere with the X-linked inhibitor of apoptosis (XIAP)-imposed inhibition of caspases (4). In addition, IAP inhibitors trigger proteasomal degradation of cIAP proteins by stimulating their E3 ligase activity (5, 6). Depletion of cIAP proteins favors the deubiquitination of receptor-activating protein 1 (RIP1), which in turn forms together with FADD and caspase-8 a platform that promotes caspase-8 activation (7, 8). Degradation of cIAP proteins also engages the non-canonical NF-κB pathway via stabilization of NF-κB-inducing kinase, which stimulates the production of prototype NF-κB target genes such as TNFα (5, 6). During IAP inhibitor-induced apoptosis, TNFα has been reported to promote apoptosis in an autocrine/paracrine loop (5, 6).
Rhabdomyosarcoma (RMS) is the most frequent pediatric soft tissue sarcoma (9). There are two most common pathological subtypes, i.e. embryonal and alveolar (9). The prognosis for children with RMS is still poor irrespective of aggressive multimodal treatment protocols (10), underscoring the need for innovative therapeutic approaches. Searching for novel strategies to trigger apoptotic cell death in RMS cells, we evaluated two agonistic TRAIL receptor (TRAIL-R)-specific antibodies against TRAIL-R1 and TRAIL-R2, i.e. mapatumumab and lexatumumab, alone and in combination with small-molecule IAP inhibitors.
EXPERIMENTAL PROCEDURES
Cell Culture and Chemicals
RMS cell lines were obtained from the American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 medium or DMEM (Invitrogen) supplemented with 10% FCS (Biochrom, Berlin, Germany), 1 mm glutamine (Invitrogen), 1% penicillin/streptomycin (Invitrogen), and 25 mm HEPES (Biochrom). N-Benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (Z-VAD-fmk) was purchased from Bachem (Heidelberg, Germany), recombinant human TNFα from Biochrom, necrostatin-1 from Biomol GmbH (Hamburg, Germany), and all chemicals from Sigma unless indicated otherwise. Enbrel was kindly provided by Pfizer. The fully human agonist monoclonal antibodies against TRAIL-R1 and TRAIL-R2, mapatumumab and lexatumumab, respectively, were kind gifts from Human Genome Sciences, Inc. (Rockville, MD) (11). IAP inhibitor 2 corresponds to compound 11 described by Oost et al. (12), and IAP inhibitor 3 was described by Chao et al. (13), and they were kindly provided by Idun Pharmaceuticals (now Pfizer).
RNA Interference
For stable gene knockdown, shRNA targeting RIP1 sequence (ccactagtctgacggataa) or a control sequence with no corresponding part in the human genome (gatcatgtagatacgctca) was cloned into pGreenPuro, and lentivirus-containing supernatants were generated as described previously (14). Stable cell lines were produced by selection with 1 μg/ml puromycin (Sigma).
Determination of Apoptosis, Cell Viability, and Colony Formation
Apoptosis was determined by fluorescence-activated cell sorting analysis (FACSCanto II cytometer, BD Biosciences) of DNA fragmentation of propidium iodide-stained nuclei as described previously (15). Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer's instructions (Roche Diagnostics). For colony assay, cells were seeded as single cells (200 cells/well) in 6-well plates for 24 h and treated for 48 h before the medium was exchanged with fresh drug-free medium, and cells were cultured for an additional 10 days before staining with 0.75% crystal violet, 50% ethanol, 0.25% NaCl, and 1.57% formaldehyde.
Western Blot Analysis
Western blot analysis was performed as described previously (15) using the following antibodies: mouse anti-caspase-8 (Alexis Biochemicals, Grünberg, Germany), mouse anti-FADD and mouse anti-XIAP (clone 28) (BD Transduction Laboratories), rabbit anti-caspase-3 and mouse anti-caspase-9 (Cell Signaling, Beverly, MA), rabbit anti-TRAIL-R2 (Chemicon, Billerica, MA), goat anti-cIAP1 (R&D Systems, Wiesbaden, Germany), and rabbit anti-cIAP2 (Epitomics, Burlingame, CA). Mouse anti-GAPDH (HyTest Ltd., Turku, Finland) and mouse anti-β-actin (Sigma) antibodies were used as loading controls. Horseradish peroxidase-conjugated goat anti-mouse IgG, donkey anti-goat IgG, and goat anti-rabbit IgG (Santa Cruz Biotechnology) and horseradish peroxidase-conjugated goat anti-mouse IgG1 and goat anti-mouse IgG2b (Southern Biotech, Birmingham, AL) were used as secondary antibodies. Enhanced chemiluminescence (Amersham Biosciences) was used for detection. Representative blots of at least two independent experiments are shown.
Immunoprecipitation
Immunoprecipitation of caspase-8 was performed as described previously (14). Briefly, cells were lysed in Nonidet P-40 buffer (10 mm Tris (pH 8.0), 150 mm NaCl, and 1% Nonidet P-40 supplemented with one protease inhibitor tablet (Roche Diagnostics)). 1 mg of protein was incubated with 10 μg of mouse anti-caspase-8 antibody overnight at 4 °C, followed by the addition of 20 μl of Dynabeads pan-mouse IgG (Invitrogen), and then incubated for 2 h at 4 °C and washed with Nonidet P-40 buffer. Caspase-8 was detected using rabbit anti-caspase-8 monoclonal antibody (Epitomics), and RIP1 or FADD was detected with mouse anti-RIP antibody (BD Biosciences) or mouse anti-FADD antibody, respectively.
Cell Surface Staining
To determine surface expression of TRAIL receptors, cells were incubated with mouse anti-TRAIL-R1–R4 antibodies (10 μg/ml; all from Alexis Biochemicals) for 30 min at 4 °C, washed with PBS containing 1% FCS, incubated with biotin-conjugated rabbit anti-mouse F(ab′)2 IgG (5 μg/ml; BD Biosciences) for 20 min at 4 °C in the dark, washed with PBS containing 1% FCS, incubated with phycoerythrin-conjugated streptavidin (0.25 μg/ml; BD Biosciences) for 20 min at 4 °C in the dark, and analyzed by flow cytometry.
Determination of Mitochondrial Membrane Potential
To determine the mitochondrial membrane potential (MMP), cells were incubated with tetramethylrhodamine methyl ester (1 μm; Molecular Probes) for 30 min at 37 °C and immediately analyzed by flow cytometry.
Statistical Analysis
Statistical significance was assessed by Student's t test (two-tailed distribution, two-sample, unequal variance). Interaction between IAP inhibitors and lexatumumab was analyzed by the combination index method based on that described by Chou (16) using CalcuSyn software (Biosoft, Cambridge, United Kingdom). A combination index of <0.9 indicates synergism, 0.9–1.1 indicates additivity, and >1.1 indicates antagonism.
RESULTS
IAP Inhibitors Sensitize RMS Cells to Lexatumumab-induced Apoptosis
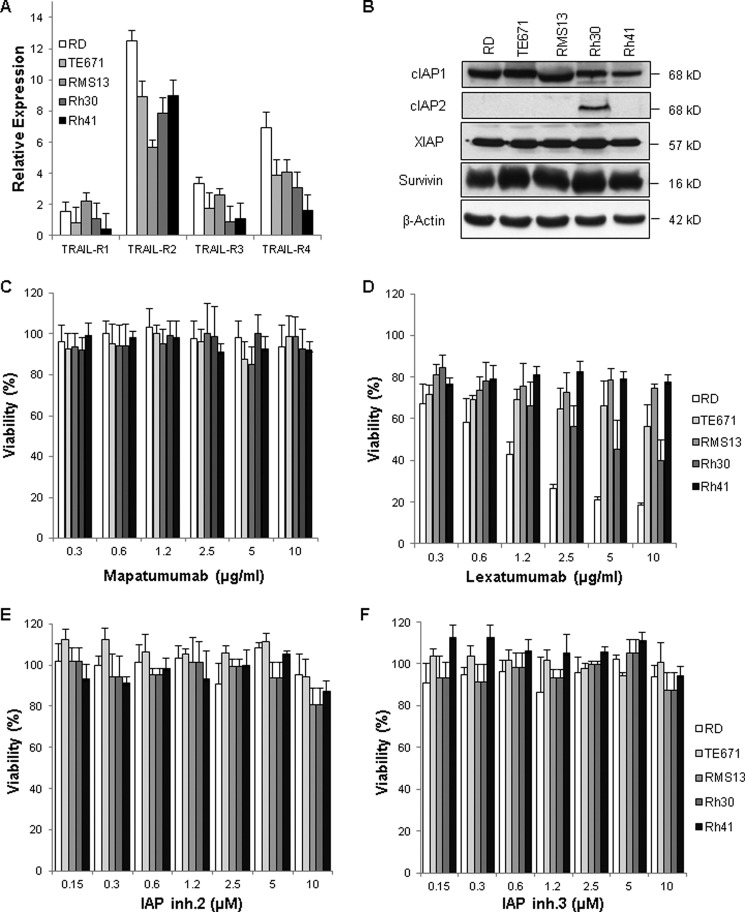
In a first approach, we examined the expression levels of TRAIL receptors and IAP proteins in several RMS cell lines, which represent the two major subtypes of RMS, i.e. embryonal RMS (RD and TE671) and alveolar RMS (RMS13, Rh30, and Rh41). RMS cell lines predominately expressed TRAIL-R2 and also displayed variable levels of TRAIL-R3 and TRAIL-R4, whereas low levels of TRAIL-R1 were detected (Fig. 1A). The predominant expression of TRAIL-R2 compared with TRAIL-R1 in RMS cell lines is consistent with a recent report (17). Expression of cIAP1, XIAP, and survivin was detected in all analyzed cell lines, whereas cIAP2 protein was detected only in Rh30 cells (Fig. 1B).
FIGURE 1.
Expression of apoptosis-signaling proteins in RMS cells and effect of mapatumumab, lexatumumab, and IAP inhibitor 3 on cell viability. A, cell surface expression of TRAIL-R1–R4 was determined by flow cytometry in RMS cell lines. The expression levels of TRAIL receptors are shown relative to the isotype control. Means ± S.D. of three independent experiments performed in triplicate are shown. B, the expression levels of cIAP1, cIAP2, XIAP, and survivin were assessed by Western blot analysis in RMS cell lines. β-Actin was used as a loading control. Representative blots of two independent experiments are shown. C–F, RMS cell lines were treated for 48 h with the indicated concentrations of mapatumumab (C), lexatumumab (D), IAP inhibitor 2 (IAP inh.2; E), and IAP inhibitor 3 (IAP inh.3; F). Cell viability was determined by MTT assay and is expressed as a percentage of untreated controls. Means ± S.D. of three independent experiments performed in triplicate are shown.
In the next step, we examined the responsiveness of RMS cell lines to fully human monoclonal antibodies that specifically bind to TRAIL-R1 (i.e. mapatumumab) or TRAIL-R2 (i.e. lexatumumab). Mapatumumab exerted little effect on the cell viability of RMS cell lines even at a relatively high concentration (10 μg/ml) (Fig. 1C). By comparison, lexatumumab decreased cell viability in a dose-dependent manner in RD, Rh30, and TE671 cells, but not in RMS13 and Rh41 cells (Fig. 1D). There was no direct correlation between the responsiveness of RMS cell lines to lexatumumab and TRAIL-R2 surface levels (Fig. 1, A and D). These findings are in line with our previous results obtained in pancreatic carcinoma cell lines (18) and with a recent study on RMS (17). This suggests that other factors in addition to TRAIL receptor surface expression determine the responsiveness of RMS cells to anti-TRAIL receptor antibodies. Furthermore, we tested the effect of small-molecule IAP inhibitors on cell viability. RMS cell lines failed to respond to single-agent treatment with two different IAP inhibitors that mimic the N-terminal tetrapeptide motif of Smac (Fig. 1, E and F).
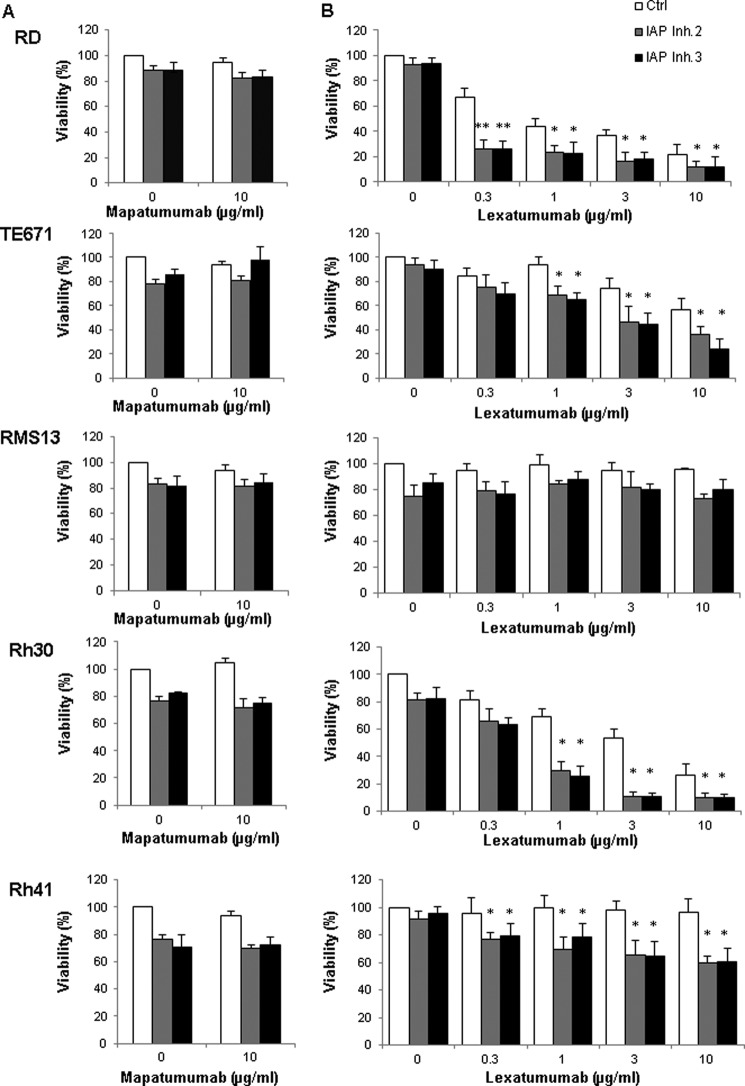
We then investigated whether IAP inhibitors are able to sensitize RMS cells to TRAIL receptor agonists. Interestingly, we found that the addition of either of the two IAP inhibitors significantly enhanced lexatumumab-induced loss of cell viability in a concentration-dependent manner in RD, TE671, Rh30, and Rh41 cells, whereas little cooperative interaction was observed in RMS13 cells (Fig. 2B). In contrast, both IAP inhibitors failed to potentiate mapatumumab-induced cytotoxicity (Fig. 2A).
FIGURE 2.
IAP inhibitors sensitize RMS cells to lexatumumab, but not to mapatumumab. RMS cell lines were treated with the indicated concentrations of mapatumumab (A), lexatumumab (B), and/or 10 μm IAP inhibitors (IAP Inh) for 48 h. Cell viability was determined by MTT assay and is expressed as a percentage of untreated controls. Means ± S.D. of three independent experiments performed in triplicate are shown. *, p < 0.05; **, p < 0.001 comparing cells treated with lexatumumab in the absence and presence of IAP inhibitors.
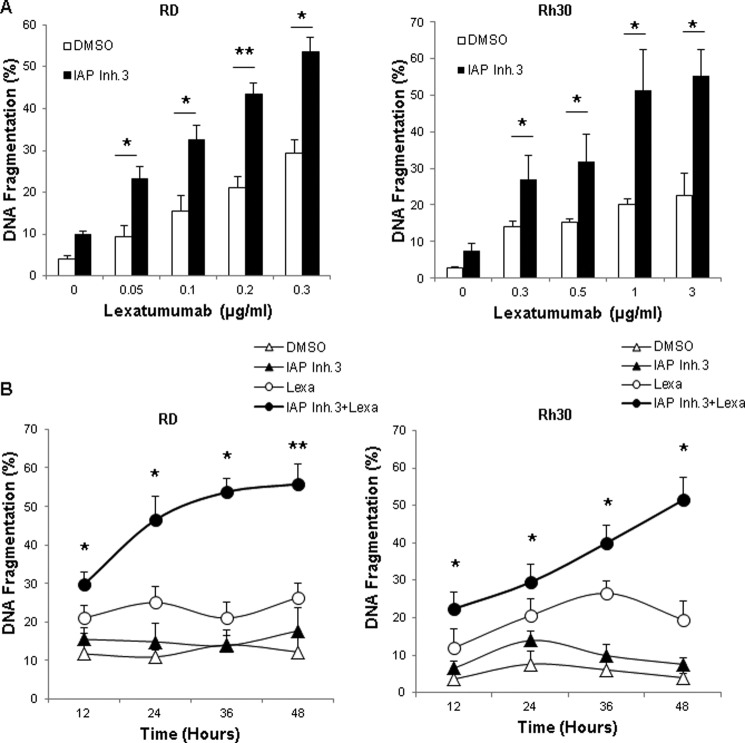
To explore in more detail the molecular mechanisms of the cooperative interaction of IAP inhibitors and lexatumumab, we selected the cell lines RD and Rh30 to represent embryonal RMS and alveolar RMS as the two major subtypes of RMS. In addition, we focused on IAP inhibitor 3 because we obtained comparable results with the two IAP inhibitors. To investigate whether cells die via apoptotic cell death, we analyzed DNA fragmentation as a hallmark of apoptosis. Of note, IAP inhibitor 3 significantly increased lexatumumab-induced DNA fragmentation in a dose-dependent fashion (Fig. 3A). Calculation of the combination index confirmed that the interaction of IAP inhibitor 3 and lexatumumab to induce apoptosis is highly synergistic (supplemental Table 1). Kinetic studies showed that IAP inhibitor 3 cooperated with lexatumumab to trigger apoptosis in a time-dependent manner (Fig. 3B). Together, this set of experiments demonstrates that IAP inhibitors sensitize several RMS cell lines to lexatumumab-induced apoptosis in a synergistic manner.
FIGURE 3.
IAP inhibitor 3 and lexatumumab cooperate to induce apoptosis. RD and Rh30 cells were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 (IAP Inh.3) and/or the indicated concentrations of lexatumumab for 48 h (A) or with 0.2 μg/ml (RD) or 1 μg/ml (Rh30) lexatumumab (Lexa) and/or 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 for the indicated time points (B). Apoptosis was determined by FACS analysis of DNA fragmentation of propidium iodide-stained nuclei. Means ± S.D. of three independent experiments performed in triplicate are shown. *, p < 0.05; **, p < 0.001 comparing cells treated with lexatumumab in the absence and presence of IAP inhibitor 3. DMSO, dimethyl sulfoxide.
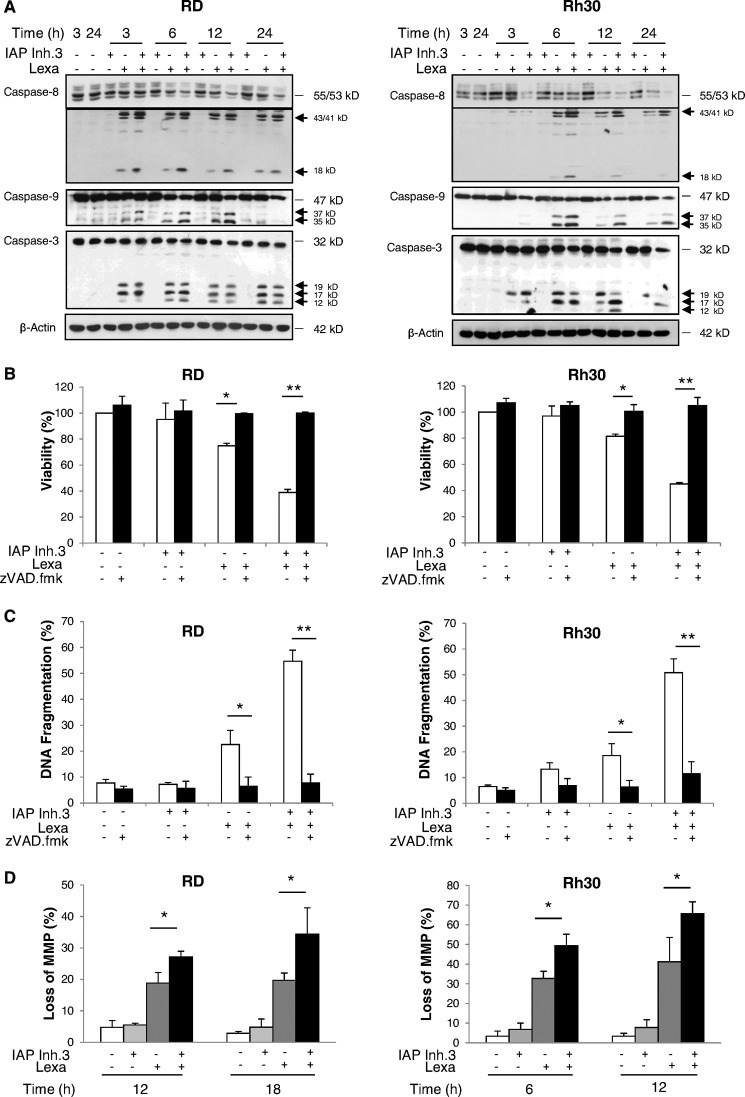
IAP Inhibitor Cooperates with Lexatumumab to Trigger Caspase Activation, Mitochondrial Perturbation, and Caspase-dependent Apoptosis
We then analyzed the effect of IAP inhibitor 3 on lexatumumab-triggered signaling events. Monitoring of caspase activation by Western blot analysis showed that IAP inhibitor 3 acted in concert with lexatumumab to induce cleavage of caspases into active fragments, including cleavage of caspase-8 into p43/p41/p18 fragments, cleavage of caspase-9 into p37/p35 fragments, and cleavage of caspase-3 into p17/p12 fragments (Fig. 4A). Also, the proenzyme forms of caspase-8, -9, and -3 decreased in cotreated cells (Fig. 4A), further supporting the notion that caspases are proteolytically turned over. To explore whether cell death depends on activation of caspases, we tested the effect of the wide-spectrum caspase inhibitor Z-VAD-fmk. The addition of Z-VAD-fmk profoundly reduced IAP inhibitor- and lexatumumab-induced apoptosis and rescued loss of cell viability (Fig. 4, B and C), demonstrating that cell death occurs in a caspase-dependent manner.
FIGURE 4.
IAP inhibitor 3 and lexatumumab cooperate to trigger caspase activation, mitochondrial perturbation, and caspase-dependent apoptosis. A, RD and Rh30 cells were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 (IAP Inh.3) and/or 0.2 μg/ml (RD) or 1 μg/ml (Rh30) lexatumumab (Lexa) for the indicated times. Caspase activation was assessed by Western blotting. Arrows indicate caspase cleavage fragments. B and C, RD and Rh30 cells were treated for 48 h with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 and/or 0.2 μg/ml (RD) or 1 μg/ml (Rh30) lexatumumab in the presence or absence of 20 μm Z-VAD-fmk. Cell viability was determined by MTT assay and is expressed as a percentage of untreated controls (B). Apoptosis was determined by FACS analysis of DNA fragmentation of propidium iodide-stained nuclei (C). D, RD and Rh30 cells were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 and/or 0.2 μg/ml (RD) or 1 μg/ml (Rh30) lexatumumab for the indicated times. Loss of MMP was analyzed by flow cytometry using the dye tetramethylrhodamine methyl ester. The percentage of live cells with loss of MMP is shown. In B–D, means ± S.D. of three independent experiments performed in triplicate are shown. *, p < 0.05; **, p < 0.001.
To investigate the effect of the combination treatment on mitochondrial functions, we assessed the MMP. IAP inhibitor 3 significantly enhanced lexatumumab-induced loss of MMP compared with treatment with lexatumumab alone in a time-dependent fashion (Fig. 4D). Because apoptosis triggered by monotherapy with IAP inhibitors has been attributed to a TNFα-mediated autocrine cell death loop that drives activation of the caspase cascade (5, 6), we next tested whether the addition of the TNFα-blocking antibody Enbrel rescues cells from IAP inhibitor- and lexatumumab-induced apoptosis. However, Enbrel failed to block the combination treatment-mediated apoptosis or loss of viability (supplemental Fig. 1, A and B). By comparison, Enbrel inhibited the IAP inhibitor 3- and TNFα-triggered loss of viability that was used as a positive control (supplemental Fig. 1C). These experiments show that the IAP inhibitor promotes lexatumumab-mediated caspase activation, loss of MMP, and apoptosis in a caspase-dependent but TNFα-independent manner.
IAP inhibitor and Lexatumumab Cooperate to Trigger RIP1·FADD·Caspase-8 Complex Formation
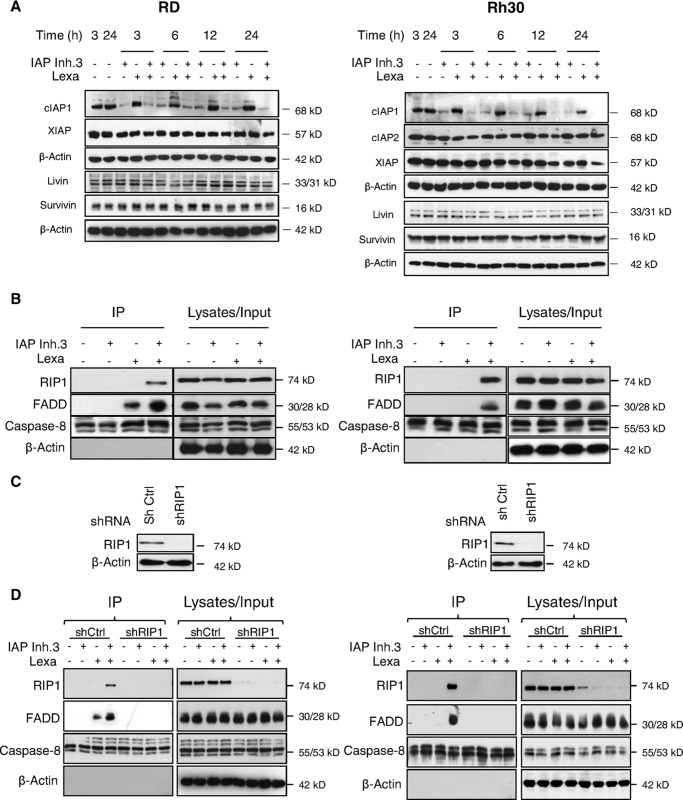
Because IAP antagonists were reported to trigger proteasomal degradation of RING domain-containing IAP proteins (5, 6), we monitored their expression levels upon treatment with IAP inhibitor 3 and/or lexatumumab. Exposure to IAP inhibitor 3 resulted in rapid and profound down-regulation of cIAP1, whereas the expression levels of XIAP and cIAP2 were slightly reduced at later time points in response to the combination treatment (Fig. 5A). cIAP2 expression was monitored only in Rh30 cells because RD cells express little cIAP2 protein (Fig. 1B).
FIGURE 5.
IAP inhibitor 3 and lexatumumab cooperate to trigger formation of the RIP1·FADD·caspase-8 complex. A, RD and Rh30 cells were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 (IAP Inh.3) and/or 0.2 μg/ml (RD) or 1 μg/ml (Rh30) lexatumumab (Lexa) for the indicated times. Expression of cIAP1, cIAP2, and XIAP was assessed by Western blotting. B, RD and Rh30 cells were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 and/or 0.2 μg/ml (RD) or 1 μg/ml (Rh30) lexatumumab for 1 h. Caspase-8 was immunoprecipitated (IP) using an anti-caspase-8 antibody, and the indicated proteins were detected by Western blot analysis. C and D, RD and Rh30 cells were transduced with control vector (shCtrl) or a vector containing a shRNA sequence against RIP1 (shRIP1). Expression of RIP1 was analyzed by Western blotting (C). Cells were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 and/or 0.2 μg/ml (RD) or 0.3 μg/ml (Rh30) lexatumumab for 1 h. Caspase-8 was immunoprecipitated using an anti-caspase-8 antibody, and the indicated proteins were detected by Western blot analysis (D).
Because depletion of cIAP proteins has been described to result in RIP1 deubiquitination, which in turn favors the assembly of a cytosolic complex containing RIP1, FADD, and caspase-8 (7, 19), we next examined the formation of this complex by immunoprecipitation of caspase-8. Interestingly, IAP inhibitor and lexatumumab acted together to stimulate the assembly of the RIP1·FADD·caspase-8 complex, whereas no or incomplete complex formation was observed upon treatment with either agent alone (Fig. 5B).
RIP1 Is Required for IAP Inhibitor- and Lexatumumab-induced Apoptosis
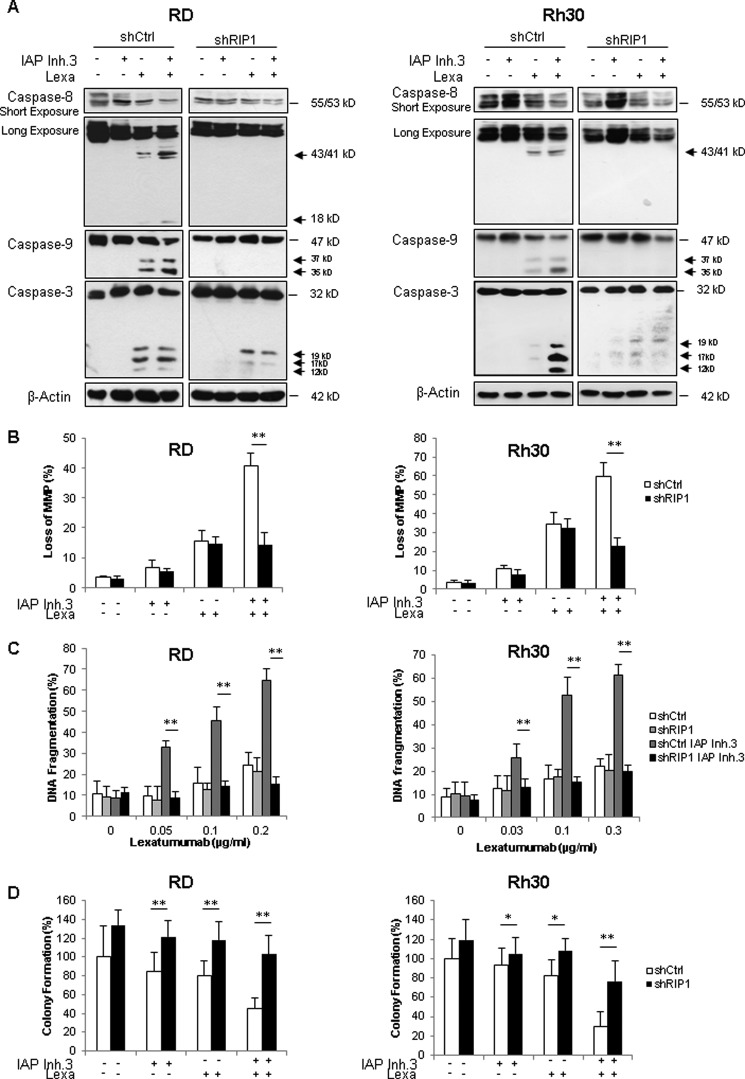
To examine whether RIP1 is required for IAP inhibitor- and lexatumumab-induced apoptosis, we genetically silenced RIP1 by RNAi (Fig. 5C). Of note, knockdown of RIP1 completely prevented the IAP inhibitor- and lexatumumab-stimulated assembly of the RIP1·FADD·caspase-8 complex (Fig. 5D). Also, RIP1 silencing inhibited activation of caspase-8, -9, and -3 as well as loss of MMP upon the combination treatment with IAP inhibitor and lexatumumab (Fig. 6, A and B). Importantly, RIP1 silencing profoundly inhibited IAP inhibitor- and lexatumumab-induced DNA fragmentation and loss of cell viability in both RMS cell lines (Fig. 6C and supplemental Fig. 2). In addition, RIP1 knockdown rescued clonogenic survival of cells treated with the combination of lexatumumab and IAP inhibitor (Fig. 6D), thus pointing to a critical role of RIP1 in cotreatment-induced apoptosis.
FIGURE 6.
RIP1 is required for IAP inhibitor 3- and lexatumumab-induced caspase activation, mitochondrial perturbation, and apoptosis. A, cells transduced with control vector (shCtrl) or a vector containing a shRNA sequence against RIP1 (shRIP1) were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 (IAP Inh.3) and/or 0.2 μg/ml (RD) or 0.3 μg/ml (Rh30) lexatumumab (Lexa) for 6 h. Caspase activation was assessed by Western blotting. Cleavage fragments are indicated by arrows. B, cells transduced with control vector (white bars) or a vector containing a shRNA sequence against RIP1 (black bars) were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 and/or 0.2 μg/ml (RD) or 0.3 μg/ml (Rh30) lexatumumab for 18 h (RD) or 12 h (Rh30), and loss of MMP was analyzed by flow cytometry using tetramethylrhodamine methyl ester dye. The percentage of live cells with loss of MMP is shown. C, cells transduced with control vector or a vector containing a shRNA sequence against RIP1 were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 and/or the indicated concentrations of lexatumumab for 48 h. Apoptosis was determined by FACS analysis of DNA fragmentation of propidium iodide-stained nuclei. D, cells were treated with 1 μm (RD) or 2.5 μm (Rh30) IAP inhibitor 3 and/or 0.2 μg/ml (RD) or 0.3 μg/ml (Rh30) lexatumumab for 48 h before the medium was exchanged with fresh drug-free medium, and cells were cultured for an additional 10 days before staining with crystal violet solution. Colonies were counted, and colony formation is expressed as a percentage of untreated controls. In B–D, means ± S.D. of three independent experiments performed in triplicate (B and C) or uniplicate (D) are shown. *, p < 0.05; **, p < 0.001.
In addition to this genetic strategy, we also employed a pharmacological approach to inhibit the kinase activity of RIP1 using the allosteric RIP1 inhibitor necrostatin-1 (20). Necrostatin-1 slightly inhibited IAP inhibitor- and lexatumumab-induced apoptosis in RD cells, whereas it did not interfere with IAP inhibitor- and lexatumumab-induced apoptosis or loss of cell viability in Rh30 cells (supplemental Fig. 3), indicating that RIP1 kinase activity is largely dispensable for the combination treatment-triggered apoptosis. Together, this set of experiments demonstrates that IAP inhibitor and lexatumumab act together to trigger the formation of a RIP1·FADD·caspase-8 complex, which drives activation of the caspase cascade, mitochondrial perturbation, and caspase-dependent apoptosis.
DISCUSSION
Searching for new strategies to trigger apoptosis in RMS, we explored the effect of two novel classes of apoptosis-targeting agents, i.e. anti-TRAIL receptor antibodies such as mapatumumab and lexatumumab and small-molecule inhibitors of IAP proteins. Here, we report for the first time that IAP inhibitors represent a promising strategy to potentiate the antitumor activity of the TRAIL-R2 agonist lexatumumab in RMS. This conclusion is supported by several independent lines of evidence. IAP inhibitors act in concert with lexatumumab to induce apoptosis in several RMS cells. This interaction occurs in a highly synergistic fashion as demonstrated by a combination index of <0.1. The finding that IAP inhibitors synergize with lexatumumab, but not with mapatumumab, to induce apoptosis may be related to the predominant expression of TRAIL-R2 and very low TRAIL-R1 expression in RMS cells. A recent study similarly reported that TRAIL-R1 is present at low levels in RMS cell lines, although the underlying molecular mechanisms were not identified (17). As epigenetic events have been described to regulate the expression of apoptosis regulatory proteins, including TRAIL receptors and caspase-8 (21, 22), it will be interesting to explore whether epigenetic silencing of TRAIL-R1 may contribute to its low expression in RMS.
Mechanistically, IAP inhibitor and lexatumumab cooperate to trigger the formation of a cytosolic complex containing RIP1, FADD, and caspase-8, which constitutes a critical platform for activation of caspase-8 and subsequent activation of apoptosis signaling pathways, including mitochondrial outer membrane permeabilization, activation of caspase-9, full activation of caspase-3, and caspase-dependent apoptosis. Accordingly, silencing of RIP1 by RNAi inhibits the combination treatment-stimulated assembly of the RIP1·FADD·caspase-8 complex and the resulting activation of caspases, loss of MMP, and apoptosis. Importantly, RIP1 knockdown also rescues clonogenic survival of cells treated with the combination of lexatumumab and IAP inhibitor, thus underscoring the critical role of RIP1 in cotreatment-induced apoptosis. In contrast, an autocrine TNFα loop turns out to be dispensable for IAP inhibitor/lexatumumab-induced apoptosis.
By demonstrating that RIP1 is a critical mediator of the synergism of IAP inhibitors and lexatumumab in RMS cells, our findings contribute to the growing body of evidence that RIP1 plays an important role in controlling cell death in response to depletion of IAP proteins. Because cIAP proteins act as E3 ligases that put Lys-63-linked ubiquitin chains onto RIP1, IAP inhibitor-stimulated proteasomal degradation of cIAP proteins leads to deubiquitination of RIP1, thereby promoting the interaction of RIP1 with FADD and caspase-8 in the cytosol (7, 19, 23). In this study, we have demonstrated that this RIP1·FADD·caspase-8 complex is required for the synergistic induction of apoptosis by IAP inhibitor together with the anti-TRAIL-R2 agonistic antibody lexatumumab. RIP1 has previously been identified as a critical mediator of apoptosis following treatment with IAP antagonists alone or in combination with other cell death stimuli, including TNFα, anti-CD95 agonistic antibodies, DNA-damaging drugs, and Toll-like receptor activation (7, 14, 23–28).
In addition to apoptosis, RIP1 has been identified as a key regulator of necroptosis (29). In this context, the kinase activity of RIP1 has been shown to be crucial for necroptosis induction by phosphorylating RIP3, although some forms of regulated necrosis independent of RIP1 kinase activity have also been described (30). In this study, we found that RIP1 kinase activity is largely dispensable for IAP inhibitor/lexatumumab-induced apoptosis, whereas RIP1 protein expression is required because knockdown of RIP1 by RNAi significantly reduced IAP inhibitor/lexatumumab-induced apoptosis, whereas necrostatin-1 had little or no effect. The occurrence of apoptotic rather than necroptotic cell death in this study is supported by several lines of evidence, e.g. the detection of typical features of apoptosis such as DNA fragmentation, caspase activation, loss of MMP, and rescue by the caspase inhibitor Z-VAD-fmk. This indicates that the expression of RIP1 protein is required to mediate the assembly of the RIP1·FADD·caspase-8 complex, which drives caspase-8 activation, mitochondrial perturbation, and full activation of the caspase cascade during IAP inhibitor/lexatumumab-induced apoptosis in RMS cells. However, the requirement of RIP1 kinase activity for the induction of apoptosis may depend on the context, e.g. the stimulus and/or cell type, because we recently reported that inhibition of RIP1 kinase activity by necrostatin-1 significantly decreases IAP inhibitor- and cytarabine-induced apoptosis in acute leukemia cells (14).
In addition to these insights into the signaling pathways that are regulated by IAP inhibitors in cancer cells, our study has important implications for the design of experimental therapies for RMS that aim to activate apoptosis pathways. The fact that monotherapy with either the Smac mimetic LCL161 or the anti-TRAIL-R1 antibody mapatumumab showed little antitumor activity in the pediatric preclinical testing program (31, 32) underscores the relevance to rationally develop combination therapies to synergistically trigger apoptosis. The concept of simultaneously targeting IAP proteins and TRAIL receptors is, in principle, suitable for clinical translation because IAP antagonists and anti-TRAIL receptor antibodies have individually already entered the stage of early clinical evaluation (33, 34). In addition, we previously reported that IAP inhibitors preferentially prime various cancer types, but not non-malignant cells, to TRAIL receptor-induced apoptosis, pointing to some cancer selectivity (35–37). Thus, by demonstrating that IAP inhibitors and lexatumumab synergistically trigger apoptosis in a RIP1-dependent and TNFα-independent manner in RMS cells, our findings have important implications for the development of experimental treatment strategies for RMS. Beyond RMS, this study, together with our previous reports (18, 35–38), underscores the broader relevance of the concept of simultaneously targeting IAP proteins and TRAIL receptors as a promising anticancer strategy.
Acknowledgments
We thank C. Hulford and M. Luzzio (Pfizer) for providing IAP inhibitors and C. Hugenberg for expert secretarial assistance.
This work was supported in part by grants from the Deutsche Krebshilfe and the Federal Minisrty of Education and Research (01GM0871 and 01GM1104C) (to S. F.).

This article contains supplemental Figs. 1–3 and Table 1.
- TRAIL
- TNF-related apoptosis-inducing ligand
- Smac
- second mitochondria-derived activator of caspases
- IAP
- inhibitor of apoptosis
- XIAP
- X-linked IAP
- cIAP
- cellular IAP
- RIP
- receptor-activating protein
- RMS
- rhabdomyosarcoma
- TRAIL-R
- TRAIL receptor
- Z-VAD-fmk
- N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MMP
- mitochondrial membrane potential.
REFERENCES
- 1. Ashkenazi A. (2008) Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat. Rev. Drug Discov. 7, 1001–1012 [DOI] [PubMed] [Google Scholar]
- 2. Fulda S., Galluzzi L., Kroemer G. (2010) Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 9, 447–464 [DOI] [PubMed] [Google Scholar]
- 3. Fulda S. (2009) Tumor resistance to apoptosis. Int. J. Cancer 124, 511–515 [DOI] [PubMed] [Google Scholar]
- 4. LaCasse E. C., Mahoney D. J., Cheung H. H., Plenchette S., Baird S., Korneluk R. G. (2008) IAP-targeted therapies for cancer. Oncogene 27, 6252–6275 [DOI] [PubMed] [Google Scholar]
- 5. Varfolomeev E., Blankenship J. W., Wayson S. M., Fedorova A. V., Kayagaki N., Garg P., Zobel K., Dynek J. N., Elliott L. O., Wallweber H. J., Flygare J. A., Fairbrother W. J., Deshayes K., Dixit V. M., Vucic D. (2007) IAP antagonists induce autoubiquitination of cIAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131, 669–681 [DOI] [PubMed] [Google Scholar]
- 6. Vince J. E., Wong W. W., Khan N., Feltham R., Chau D., Ahmed A. U., Benetatos C. A., Chunduru S. K., Condon S. M., McKinlay M., Brink R., Leverkus M., Tergaonkar V., Schneider P., Callus B. A., Koentgen F., Vaux D. L., Silke J. (2007) IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 [DOI] [PubMed] [Google Scholar]
- 7. Bertrand M. J., Milutinovic S., Dickson K. M., Ho W. C., Boudreault A., Durkin J., Gillard J. W., Jaquith J. B., Morris S. J., Barker P. A. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 [DOI] [PubMed] [Google Scholar]
- 8. Varfolomeev E., Wayson S. M., Dixit V. M., Fairbrother W. J., Vucic D. (2006) The inhibitor of apoptosis protein fusion cIAP2·MALT1 stimulates NF-κB activation independently of TRAF1 and TRAF2. J. Biol. Chem. 281, 29022–29029 [DOI] [PubMed] [Google Scholar]
- 9. Dagher R., Helman L. (1999) Rhabdomyosarcoma: an overview. Oncologist 4, 34–44 [PubMed] [Google Scholar]
- 10. Hayes-Jordan A., Andrassy R. (2009) Rhabdomyosarcoma in children. Curr. Opin. Pediatr. 21, 373–378 [DOI] [PubMed] [Google Scholar]
- 11. Humphreys R. C., Halpern W. (2008) TRAIL receptors: targets for cancer therapy. Adv. Exp. Med. Biol. 615, 127–158 [DOI] [PubMed] [Google Scholar]
- 12. Oost T. K., Sun C., Armstrong R. C., Al-Assaad A. S., Betz S. F., Deckwerth T. L., Ding H., Elmore S. W., Meadows R. P., Olejniczak E. T., Oleksijew A., Oltersdorf T., Rosenberg S. H., Shoemaker A. R., Tomaselli K. J., Zou H., Fesik S. W. (2004) Discovery of potent antagonists of the anti-apoptotic protein XIAP for the treatment of cancer. J. Med. Chem. 47, 4417–4426 [DOI] [PubMed] [Google Scholar]
- 13. Chao B., Deckwerth T. L., Furth P., S., Linton S., D., Spada A., P., Ullman B., R., Weinhouse M. I. (February 16, 2006) U.S. Patent PCT/US2005/024700
- 14. Löder S., Fakler M., Schoeneberger H., Cristofanon S., Leibacher J., Vanlangenakker N., Bertrand M. J., Vandenabeele P., Jeremias I., Debatin K. M., Fulda S. (2012) RIP1 is required for IAP inhibitor-mediated sensitization of childhood acute leukemia cells to chemotherapy-induced apoptosis. Leukemia 26, 1020–1029 [DOI] [PubMed] [Google Scholar]
- 15. Fulda S., Sieverts H., Friesen C., Herr I., Debatin K. M. (1997) The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res. 57, 3823–3829 [PubMed] [Google Scholar]
- 16. Chou T. C. (1991) in Synergism and Antagonism in Chemotherapy (Chou T. C., ed) pp. 61–102, Academic Press, San Diego, CA [Google Scholar]
- 17. Kang Z., Chen J. J., Yu Y., Li B., Sun S. Y., Zhang B., Cao L. (2011) Drozitumab, a human antibody to death receptor 5, has potent antitumor activity against rhabdomyosarcoma with the expression of caspase-8 predictive of response. Clin. Cancer Res. 17, 3181–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stadel D., Mohr A., Ref C., MacFarlane M., Zhou S., Humphreys R., Bachem M., Cohen G., Möller P., Zwacka R. M., Debatin K. M., Fulda S. (2010) TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors. Clin. Cancer Res. 16, 5734–5749 [DOI] [PubMed] [Google Scholar]
- 19. Varfolomeev E., Goncharov T., Fedorova A. V., Dynek J. N., Zobel K., Deshayes K., Fairbrother W. J., Vucic D. (2008) cIAP1 and cIAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G. D., Mitchison T. J., Moskowitz M. A., Yuan J. (2005) Chemical inhibitor of non-apoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 [DOI] [PubMed] [Google Scholar]
- 21. Fulda S., Küfer M. U., Meyer E., van Valen F., Dockhorn-Dworniczak B., Debatin K. M. (2001) Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene 20, 5865–5877 [DOI] [PubMed] [Google Scholar]
- 22. van Noesel M. M., van Bezouw S., Voûte P. A., Herman J. G., Pieters R., Versteeg R. (2003) Clustering of hypermethylated genes in neuroblastoma. Genes Chromosomes Cancer 38, 226–233 [DOI] [PubMed] [Google Scholar]
- 23. Wang L., Du F., Wang X. (2008) TNFα induces two distinct caspase-8 activation pathways. Cell 133, 693–703 [DOI] [PubMed] [Google Scholar]
- 24. Petersen S. L., Wang L., Yalcin-Chin A., Li L., Peyton M., Minna J., Harran P., Wang X. (2007) Autocrine TNFα signaling renders human cancer cells susceptible to Smac mimetic-induced apoptosis. Cancer Cell 12, 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biton S., Ashkenazi A. (2011) NEMO and RIP1 control cell fate in response to extensive DNA damage via TNFα feedforward signaling. Cell 145, 92–103 [DOI] [PubMed] [Google Scholar]
- 26. Tenev T., Bianchi K., Darding M., Broemer M., Langlais C., Wallberg F., Zachariou A., Lopez J., MacFarlane M., Cain K., Meier P. (2011) The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 [DOI] [PubMed] [Google Scholar]
- 27. Feoktistova M., Geserick P., Kellert B., Dimitrova D. P., Langlais C., Hupe M., Cain K., MacFarlane M., Häcker G., Leverkus M. (2011) cIAPs block Ripoptosome formation, a RIP1/caspase-8-containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geserick P., Hupe M., Moulin M., Wong W. W., Feoktistova M., Kellert B., Gollnick H., Silke J., Leverkus M. (2009) Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J. Cell Biol. 187, 1037–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hitomi J., Christofferson D. E., Ng A., Yao J., Degterev A., Xavier R. J., Yuan J. (2008) Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135, 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vanlangenakker N., Vanden Berghe T., Vandenabeele P. (2012) Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 19, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith M. A., Morton C. L., Kolb E. A., Gorlick R., Keir S. T., Carol H., Lock R., Kang M. H., Reynolds C. P., Maris J. M., Watkins A. E., Houghton P. J. (2010) Initial testing (stage 1) of mapatumumab (HGS-ETR1) by the pediatric preclinical testing program. Pediatr. Blood Cancer 54, 307–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Houghton P. J., Kang M. H., Reynolds C. P., Morton C. L., Kolb E. A., Gorlick R., Keir S. T., Carol H., Lock R., Maris J. M., Billups C. A., Smith M. A. (2012) Initial testing (stage 1) of LCL161, a Smac mimetic, by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 58, 636–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fox N. L., Humphreys R., Luster T. A., Klein J., Gallant G. (2010) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor 1 and receptor 2 agonists for cancer therapy. Expert Opin. Biol. Ther. 10, 1–18 [DOI] [PubMed] [Google Scholar]
- 34. Fulda S., Vucic D. (2012) Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 11, 109–124 [DOI] [PubMed] [Google Scholar]
- 35. Vogler M., Walczak H., Stadel D., Haas T. L., Genze F., Jovanovic M., Bhanot U., Hasel C., Möller P., Gschwend J. E., Simmet T., Debatin K. M., Fulda S. (2009) Small-molecule XIAP inhibitors enhance TRAIL-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 69, 2425–2434 [DOI] [PubMed] [Google Scholar]
- 36. Loeder S., Zenz T., Schnaiter A., Mertens D., Winkler D., Döhner H., Debatin K. M., Stilgenbauer S., Fulda S. (2009) A novel paradigm to trigger apoptosis in chronic lymphocytic leukemia. Cancer Res. 69, 8977–8986 [DOI] [PubMed] [Google Scholar]
- 37. Fakler M., Loeder S., Vogler M., Schneider K., Jeremias I., Debatin K. M., Fulda S. (2009) Small-molecule XIAP inhibitors cooperate with TRAIL to induce apoptosis in childhood acute leukemia cells and overcome Bcl-2-mediated resistance. Blood 113, 1710–1722 [DOI] [PubMed] [Google Scholar]
- 38. Abhari B. A., Cristofanon S., Kappler R., von Schweinitz D., Humphreys R., Fulda S. (2012) RIP1 is required for IAP inhibitor-mediated sensitization for TRAIL-induced apoptosis via a RIP·FADD·caspase-8 cell death complex. Oncogene, in press [DOI] [PubMed] [Google Scholar]