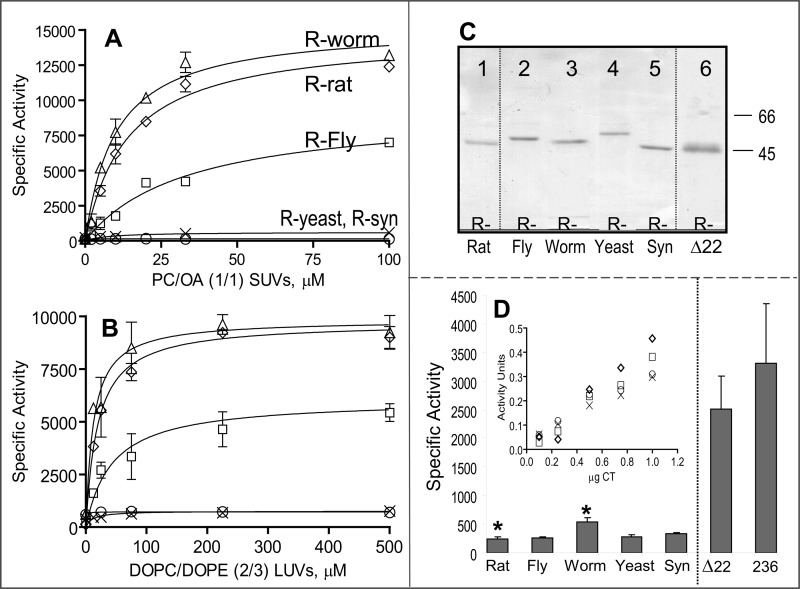
FIGURE 4.
Lipid-dependent and -independent activity of CCT chimeras. CCT was activated by egg PC/oleic acid (1:1)-sonicated vesicles (A) or DOPC/DOPE (2:3) 100 nm extruded vesicles (B). R-worm refers to the rat catalytic domain fused to the regulatory tail of the CCT from C. elegans, etc. Data are means ± S.E. of four independent determinations; in many cases, the error bar is within the symbol. Symbols in B are defined in A. C, purity of CCT chimeras. ∼2 μg of protein purified by nickel-agarose chromatography was separated on 10% SDS-PAGE and stained with Coomassie Brilliant Blue-R. Δ22 refers to rat CCTΔ272–293. D, activity of all CCT constructs in the absence of lipid. Data are means ± average deviations of 4–7 independent determinations. *, p = 0.037. Inset, micrograms of pure CCT in the assay are plotted versus activity units (nanomoles of CDP-choline formed/min) for the yeast, synuclein, and Drosophila chimeras as well as the rat control CCT. Symbols are as in A. SUV, small unilamellar vesicle; LUV, large unilamellar vesicle.