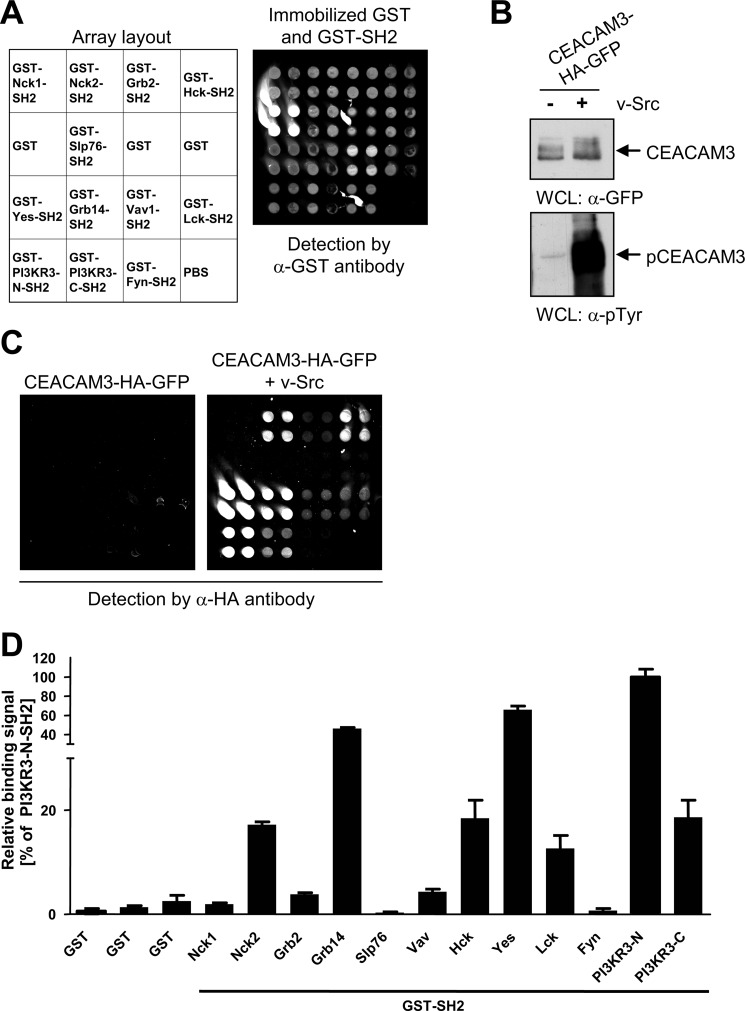
FIGURE 1.
SH2 domain microarray identifies Grb14 as an interaction partner of phosphorylated CEACAM3. A, different recombinant GST-SH2 domains, GST, or the spotting buffer (PBS) alone were immobilized in quadruplicate spots on aldehyde-modified glass slides as indicated in the array layout. Immobilized proteins were detected by monoclonal anti-GST antibody followed by Cy3-labeled secondary antibody. B, 293 cells were co-transfected with a vector encoding GFP-HA-tagged CEACAM3 together or not with v-Src. Equal amounts of whole cell lysates (WCLs) were separated by SDS-PAGE and analyzed by Western blotting with monoclonal anti-GFP (top panel), or monoclonal anti-phosphotyrosine (pTyr) (lower panel) antibodies. C, fluorescent images of SH2 arrays, probed with lysates from B. CEACAM3 bound to the array was detected by monoclonal anti-HA antibody followed by Cy3-labeled secondary antibody. D, plot shows the relative signals of phosphorylated CEACAM3 versus non-phosphorylated CEACAM3 binding to immobilized GST-fusion proteins. Bars represent mean values ± S.D. of quadruplicate spots from three independent experiments.