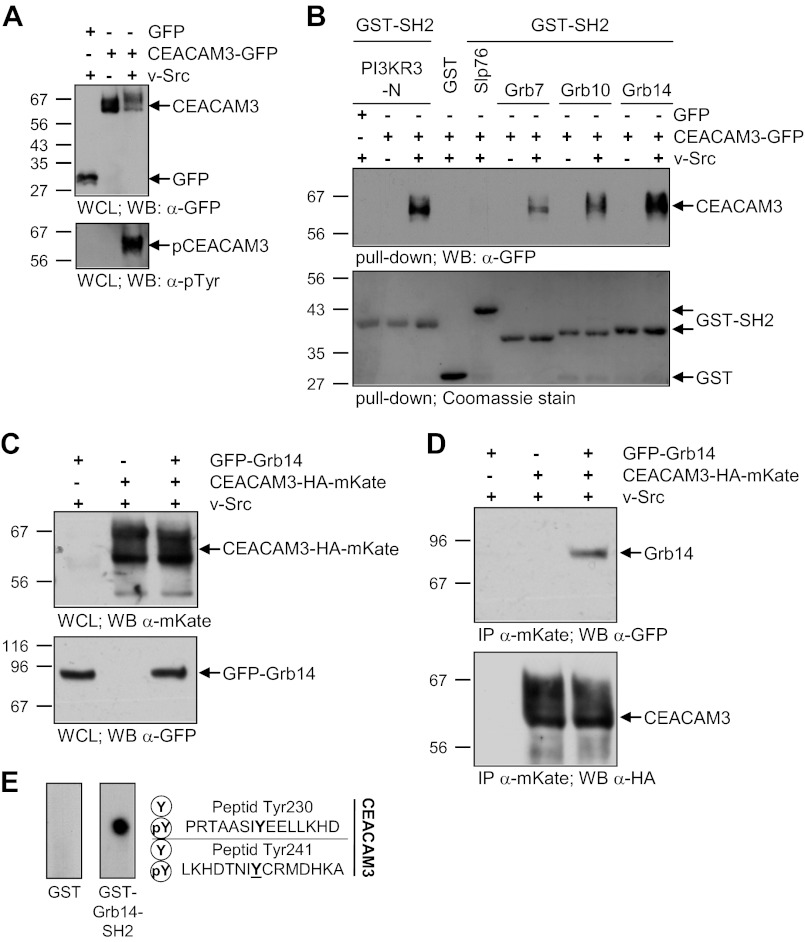
FIGURE 4.
The SH2 domain of Grb14 interacts directly with phosphorylated CEACAM3. A, 293 cells were transfected with an empty control vector (GFP) or GFP-tagged CEACAM3 together or not with v-Src. The WCLs were analyzed by Western blotting for equal expression of CEACAM3 with mAb against GFP (top panel) and tyrosine phosphorylation of CEACAM3 was verified by mAb against phosphotyrosine (pTyr; lower panel). B, indicated recombinant GST-SH2 domains or GST alone were incubated in pull-down assays with lysates from A. Precipitates were analyzed by Western blotting with monoclonal GFP antibody to detect precipitated CEACAM3 (top panel). The membranes were stained with Coomassie Brilliant Blue (Coomassie) to verify equal amounts of GST or GST-fusion proteins used in the pull-down (lower panel). C, 293 cells were co-transfected with or without a vector encoding GFP-tagged Grb14 and a vector encoding mKate-HA-tagged CEACAM3 together with v-Src. Equal amounts of whole cell lysates (WCLs) were separated by SDS-PAGE and analyzed by Western blotting with polyclonal anti-mKate (top panel) or monoclonal anti-GFP (lower panel) antibodies. D, lysates from C were used in an immunoprecipitation (IP) with pAb against mKate. Precipitates were probed with monoclonal anti-GFP antibody against GFP-Grb14 (top panel) and after stripping of the membrane, the immunoprecipitated CEACAM3-HA-mKate was detected with monoclonal anti-HA antibody (lower panel). E, peptide spot membranes harboring synthetic 15-mer peptides surrounding tyrosine residues of the CEACAM3 cytoplasmic domain (as indicated) in the unphosphorylated (Y) or the tyrosine-phosphorylated (pY) form were probed with GST or GST-Grb14-SH2. Bound GST-fusion proteins were detected with monoclonal anti-GST antibody.