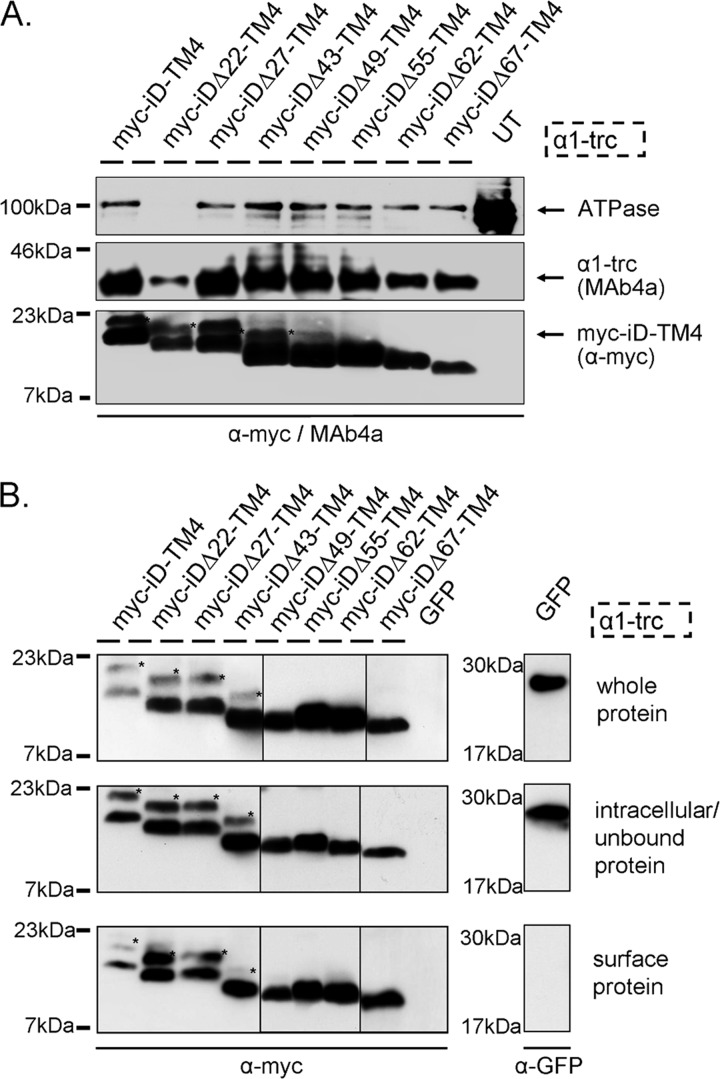
FIGURE 3.
Protein expression of GlyR domains. A, membrane protein expression is shown for α1-trc and the Myc-iDΔx-TM4 constructs. α1-trc exhibits an apparent molecular mass of 37 kDa and was stained with the pan-α antibody MAb4a, recognizing an epitope in the N-terminal portion of all GlyRα proteins. Myc-iDΔx-TM4 constructs (Δ22, Δ27, Δ43, Δ47, Δ55, Δ62, and Δ67) were detected with the α-Myc antibody binding to the 9E10 epitope at their N-terminal ends (see location in Fig. 2B). Na+,K+-ATPase (ATPase) was used as a loading control for membrane proteins (100 kDa) as well as untransfected cells (UT) as negative control. Note that the Na+,K+-ATPase band was not present in membrane preparations from α1-trc + Myc-iDΔ22-TM4. This does not inevitably mean that Myc-iDΔ22-TM4 was less expressed. Here only half of the protein amount was loaded. The expression of Myc-iDΔ22-TM4 was indistinguishable from other Myc-iDΔx-TM4 constructs in whole cell protein amount, intracellular protein, and cell surface protein; see B. B, biotinylated protein distribution within transfected HEK293 cells expressing α1-trc in combination with the indicated complementation construct. The upper blots depict the Myc-iDΔx-TM4 variants (same constructs used as in A) in the whole-cell protein fraction. The middle panels illustrate intracellular Myc-iDΔx-TM4, due to continuous overexpression in transfected HEK293 cells and a determined binding capacity of the streptavidin beads used. Myc-iDΔx-TM4 proteins, which are located at the cell surface, were detected with the lower blot (samples were applied onto two gels; lanes for Δ49, Δ55, and Δ62 come from a different gel). The negative control for surface expression, GFP, was detectable in the whole-cell protein sample and in the intracellular fraction but not in the surface pool. All blots were stained with either the pan-α GlyR antibody MAb4a or a mouse anti-GFP antibody. A and B, Myc-iDΔx-TM4 variants differ in their molecular weights according to the amino acids truncated beginning with 20 kDa for Myc-iD-TM4, detected using an α-Myc (9E10) antibody. *, reproducible construct-dependent nonspecific bands using the α-Myc antibody. These bands may result from incomplete signal peptide cleavage.