FIGURE 5.
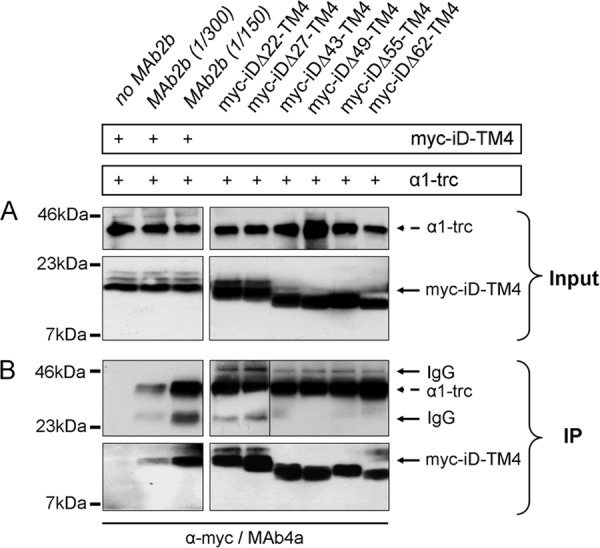
Protein-protein interactions of GlyRα1-trc with various complementation constructs. A and B, following cotransfection of α1-trc with one complementation construct, HEK293 cells were lysed (Input) and used for immunoprecipitation of α1-trc with the α1-specific GlyR antibody MAb2b binding to a native epitope in the far N-terminal portion of the α1 protein. The complementation constructs, Myc-iDΔx-TM4s, were co-immunoprecipitated (IP) and detected with a monoclonal anti-Myc (9E10) antibody. The presence of α1-trc and Myc-iD-TM4 is indicated by a plus sign. A, the input before co-immunoprecipitation of α1-trc (stained with MAb4a) and the complementation constructs Myc-iDΔx-TM4, with Δx representing Δ22, Δ27, Δ43, Δ49, Δ55, and Δ62. B, co-immunoprecipitation (IP) in a two-domain receptor configuration. The negative control without any MAb2b showed no unspecific binding (first lane, bottom panel). Increasing the amount of MAb2b (1:300 or 1:150 dilution) during the immunoprecipitation raised the specific signal for the co-precipitated Myc-iD-TM4. All complementation constructs (Myc-iDΔx-TM4) showed a strong protein-protein interaction with α1-trc. The construct Myc-iDΔ55-TM4 runs reproducibly at a higher apparent molecular weight. The IgG signals (50 and 25 kDa) became visible due to the use of two mouse monoclonal antibodies, one for precipitation (MAb2B) and the other for detection (MAb4a) of the GlyR proteins. Boxed gel lanes indicate that samples were run on different gels.
