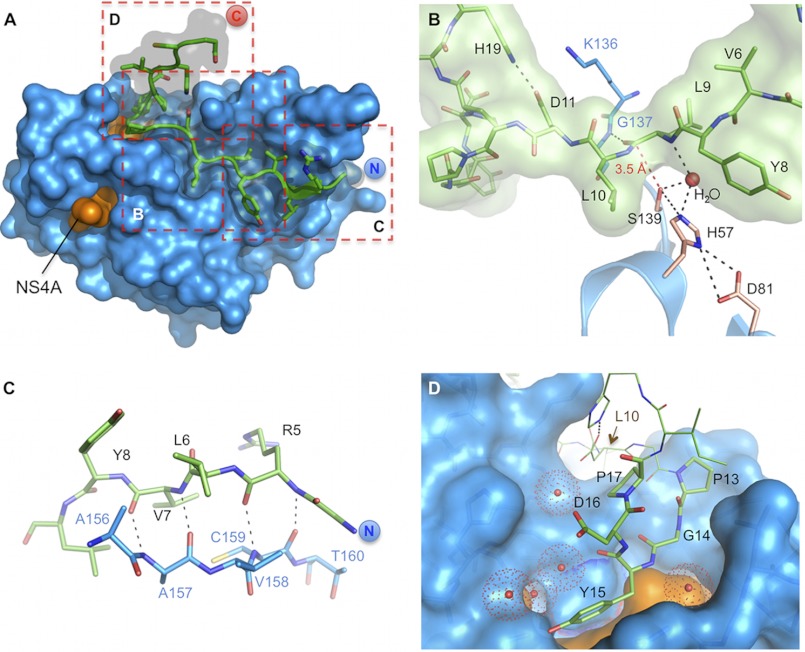
FIGURE 1.
Detailed views on the CP5-46-A/NS3-4A co-complex. A, overview on CP5-46-A (green) in complex with NS3-4A (blue/orange). Dashed red boxes indicate magnified views displayed in B–D. B, close up on NS3-4A active site (blue) with bound CP5-46-A (green). Catalytic triad residues Ser-139, His-57, and Asp-81 are colored in wheat, and other protease residues are colored in blue. A red dashed line shows the closest distance of Ser-139 and CP5-46-A backbone (Leu-9–Leu-10). H-bonds of an active site H2O are drawn with black dashed lines. C, non-primed site of the protease, CP5-46-A main chain (green) forms an anti-parallel β-strand with the backbone of NS3-4A (blue). D, primed site of NS3-4A with accommodated tyrosine finger (aa 13PGYDP17). Coordinated water molecules are shown as red spheres. The NS4A peptide is colored orange. aa 10–12 and 18–19 of CP5-46-A are shown in line representation.