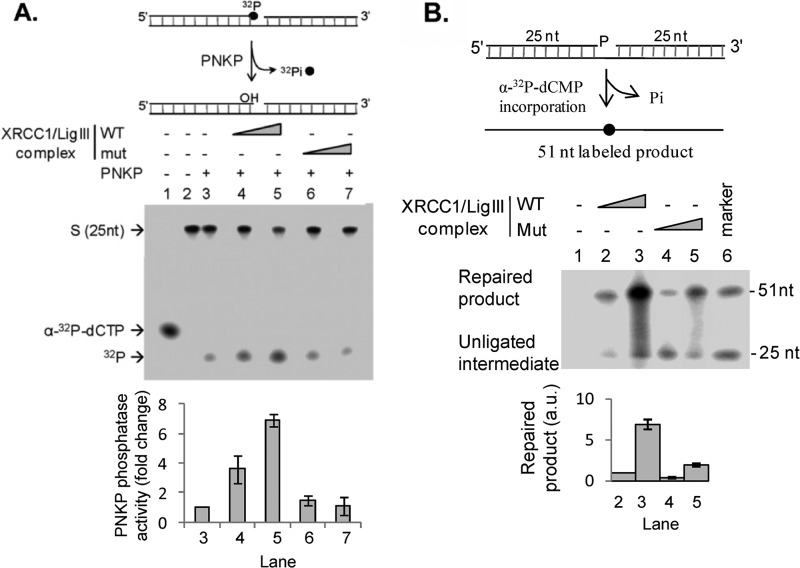
FIGURE 3.
The interaction with XRCC1 enhances the 3′-phosphatase activity of PNKP and the joining of single-strand breaks with 3′- and 5′-phosphate termini. A, the removal of labeled 3′-phosphate groups from a single strand break (upper panel and middle panel) by PNKP was measured in the absence (middle panel, lane 3) or presence of DNA ligase IIIα-XRCC1 complexes containing wild-type XRCC1 (WT, middle panel, lanes 4 and 5) and the A482T mutant version (Mut, middle panel, lanes 6 and 7) as described under “Experimental Procedures.” Lane 2, labeled DNA substrate alone. The positions of [α-32P]dCTP (lane 1), 25-mer with labeled 3′-phosphate (S), and labeled phosphate (32P) are indicated on the left. The results of three independent experiments are shown graphically with the error bars representing S.D. (lower panel). PNKP phosphatase activity is expressed as fold-change compared with the activity of PNKP alone (lane 3). B, repair of a DNA substrate containing a single nucleotide gap with 3′- and 5′-phosphate termini (upper panel). PNKP and Polβ were incubated with the DNA substrate, [α-32P]dCTP and DNA ligase IIIα-XRCC1 complexes containing either wild-type XRCC1 (WT, middle panel, lanes 2 and 3) or the A482T mutant version (Mut, middle panel, lanes 4 and 5), as described under “Experimental Procedures.” Lane 6, end-labeled 25- and 51-mer oligonucleotides (marker). The positions of the labeled unligated intermediate and repaired product are indicated on the left. The results of three independent experiments are shown graphically with the error bars representing S.D. (lower panel). The amount of repaired product is expressed as arbitrary units determined by PhosphorImager analysis.