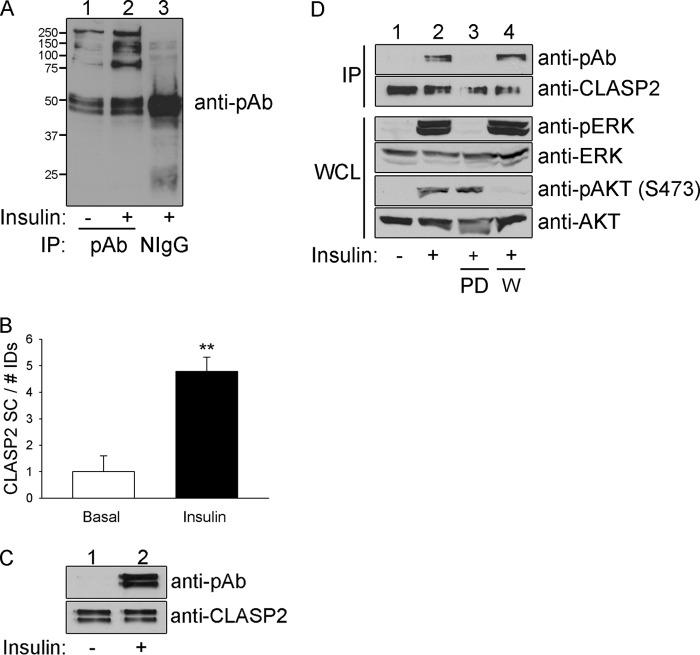
FIGURE 1.
CLASP2 undergoes insulin-stimulated phosphorylation. A, L6 myotubes were serum-starved and either left untreated or treated with 10 nm insulin for 15 min. The myotubes were lysed, and the whole cell lysates were immunoprecipitated with phosphoserine antibody. The immunoprecipitates were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes containing the immunoprecipitated proteins were subjected to Western blotting for phosphoserine in a PXS*P, S*PXR/K, or PXS*PXR/K motif. B, L6 myotubes were serum-starved and either left untreated or treated with 10 nm insulin for 15 min. The myotubes were lysed, and the whole cell lysates were immunoprecipitated with phosphoserine antibody. The immunoprecipitates were resolved by 10% SDS-PAGE followed by Coomassie staining. Each lane within the SDS-PAGE gel was divided into 15 slices, ranging the span of 250 to 15 kDa, and the proteins were prepared and analyzed as described under “Experimental Procedures.” The data were then expressed as a -fold change over basal ± S.E. (error bars; n = 3; **, p < 0.01; Student's t test). C, L6 myotubes were serum-starved and either left untreated or treated with 10 nm insulin for 15 min. The myotubes were lysed, and the whole cell lysates (WCL) were immunoprecipitated (IP) with CLASP2 antibody. The immunoprecipitates were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes containing the immunoprecipitated CLASP2 were subjected to Western blotting for phosphoserine in a PXS*P, S*PXR/K, or PXS*PXR/K motif (upper panel) or with anti-CLASP2 antibodies (lower panel). D, L6 myotubes were serum-starved and either left untreated, treated with insulin alone, or pretreated with 50 nm wortmannin (labeled W) or 50 μm PD98059 (labeled PD) for 1 h and then either left untreated or treated with 10−7 m insulin for 15 min. The myotubes were lysed, and the whole cell lysates were immunoprecipitated with CLASP2 antibody. The immunoprecipitates were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes containing the immunoprecipitated CLASP2 were subjected to Western blotting for phosphoserine in a PXS*P, S*PXR/K, or PXS*PXR/K motif (top panel) or with anti-CLASP2 antibodies (second panel from the top).Whole cell lysates were probed for the phosphorylation of either AKT (fifth panel from the top) or MAPK (third panel from the top), or the expression of AKT (bottom panel) or MAPK (fourth panel from the top).