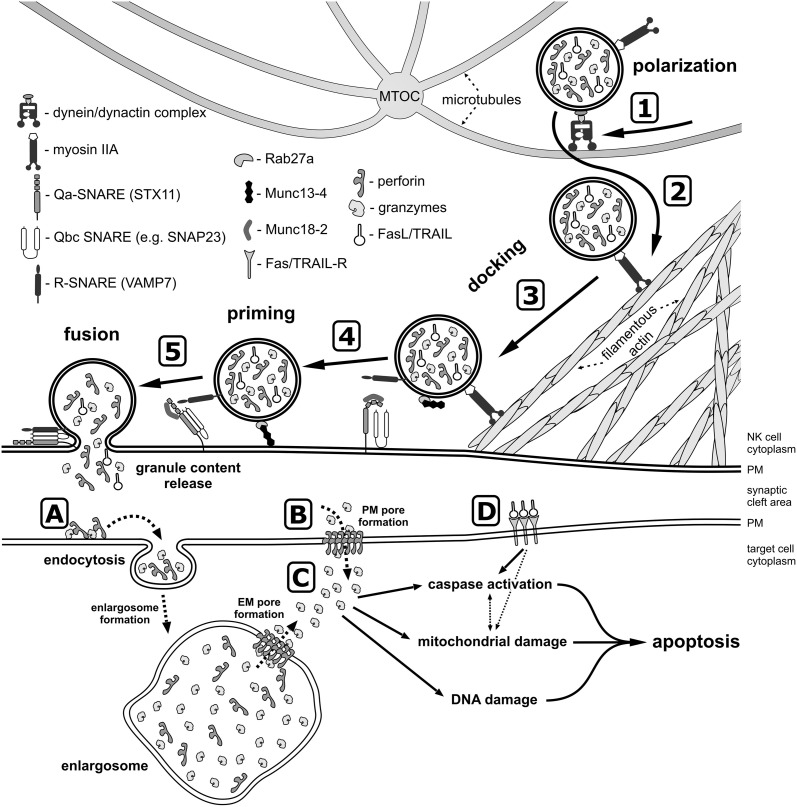
Figure 2.
A model of lytic granule exocytosis from human NK cell. In response to the engagement of NK cell activating receptors and initiation of signaling cascades (not depicted), the lytic granules move along the microtubules toward the MTOC in the dynein-dynactin complex-dependent manner (1). The MTOC and the granules then polarize toward the NK-target cell contact area, where granules switch from microtubules to the filamentous actin network at the immunological synapse (2) and navigate through the cortical filamentous actin meshwork as a result of the actin motor protein myosin IIA activity (3). This allows the lytic granules to get into close proximity of the plasma membrane (PM), and dock at the membrane due to activity of Rab27a and Rab27a-mediated recruitment of Munc13-4, as well as through the recognition of syntaxin 11 (STX11) and Munc18-2, possibly by the R-SNARE protein(s) present at the lytic granule membrane. The docked granules are then primed (4) by Munc13-4 in response to calcium flux (not shown), likely by the Munc13-4-mediated switch of STX11 to an open conformation (by removal of Munc18-2), and/or by Munc13-4 forming an initial bridge between the granule membrane and the plasma membrane. Finally, the granule-associated R-SNARE protein(s) (e.g., VAMP7) form a complex with Q-SNARE proteins present on the plasma membrane (e.g., STX11 and SNAP23) (5), which allows for the fusion of vesicles with the plasma membrane and release of the granule content into the synaptic cleft at the immunological synapse. There are two paradigms describing the entry of perforin and granzymes into target cells. The internalization model (A) assumes that perforin and granzymes bind to the target cell plasma membrane and are endocytosed into the early endosome-like enlargosome. Following internalization, perforin would mediate formation of pores in the enlargosome membrane (EM), allowing granzymes to leak into the cytosol of the target cell. According to the plasma membrane (PM) pore formation model (B), perforin oligomerizes in the plasma membrane, disrupting its integrity thereby permitting granzymes to enter from the synaptic cleft into the target cell. After gaining access into the cell cytosol (C), granzymes start processing their targets, leading to apoptosis through activation of caspases, induction of mitochondrial damage, and DNA fragmentation. In addition, FasL and TRAIL from the lytic granules bind to their receptors on the target cell surface (D) and initiate apoptosis.