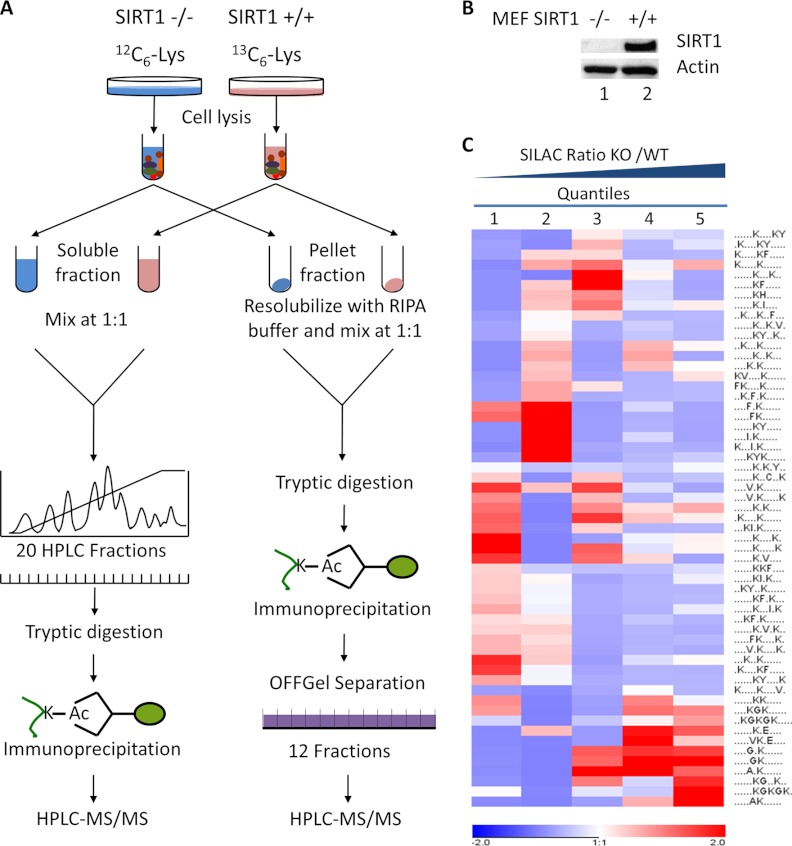
Fig. 1.
The experimental strategy for identification and quantification of Lys acetylation sites in mouse embryonic fibroblast (MEF) cells. A, MEF SIRT1+/+ (WT) and SIRT1−/− (KO) cells were grown in SILAC media with U-13C6-Lys and U-12C6-Lys respectively. Cells were sequentially lysed, generating two fractions: an NETN-soluble fraction and an NETN-pellet fraction. For each fraction, equal amounts of proteins from the two pools of cells were mixed. Proteins from NETN-soluble fractions were further resolved by ion-exchange chromatography, followed by tryptic digestion and immunoprecipitation using anti-Kac pan-antibodies. The combined proteins from NETN-pellet fractions were digested by trypsin. The resulting peptides were immunoprecipitated by anti-Kac pan-antibodies followed by OFFGel fractionation. The enriched Kac-containing peptides in each fraction were subjected to HPLC/MS/MS analysis. B, Western blot analysis of protein whole-cell lysates from MEF SIRT1+/+ and SIRT1−/− cells. C, Clustering analysis of Lysine acetylation motifs among different quantiles. Lys acetylation motifs were identified using Motif-x for all Lys acetylation sites and for sites in each quintile with Bonferroni corrected p < 0.05. HyperG test was performed for the enrichment test of each motif in all quantiles and p value was transformed into Z-score for hierarchical clustering analysis.