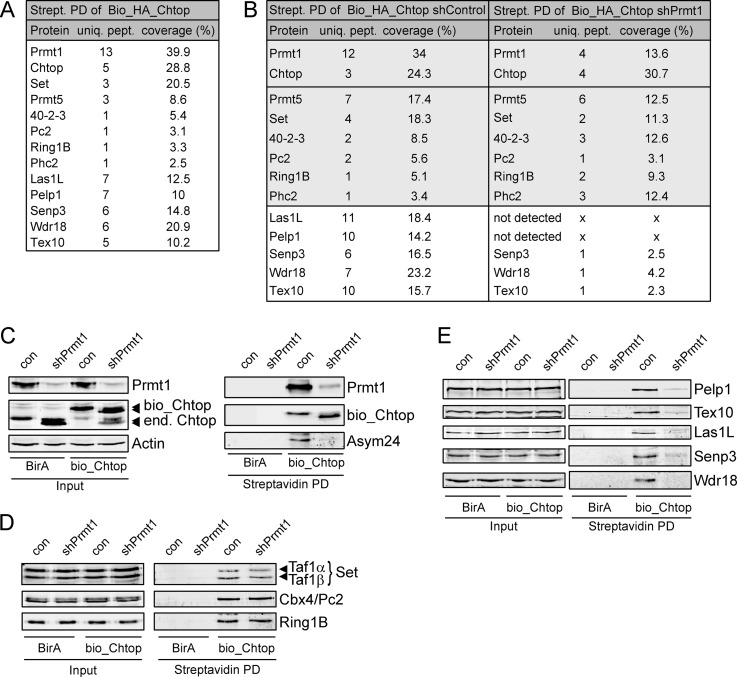
Fig. 1.
Identification of Chtop-interacting proteins. A, List of Chtop interacting proteins indentified by mass spectrometry (MS). Protein complexes from nuclear lysates of MEL_BirA cells ectopically expressing Bio_HA_Chtop were recovered by MS. Proteins, unique peptides (uniq. pept.) and percent coverage (coverage %) are indicated. B, List of Chtop interacting proteins in MEL_BirA cells treated with control lentivirus (shControl) and lentivirus expressing shRNA against Prmt1 (shPrmt1) identified by MS. Interactions with major differences between shControl and shPrmt1 treated cells are shown in a white box. C, Chtop is hypomethylated in the absence of Prmt1. Whole cell lysates (Input) and streptavidin pull downs (Streptavidin PD) were analyzed for Prmt1, Chtop and asymmetric dimethyl arginine (Asym24) residues. Actin staining serves as a loading control. Ectopically expressed (bio_Chtop) and endogenous Chtop (end. Chtop) are indicated. D, Chtop methylation-independent interactions. Whole cell lysates (Input) and streptavidin pull-downs (Streptavidin PD) were analyzed with the antibodies indicated. E, Chtop methylation-dependent interactions. Whole-cell lysates (Input) and streptavidin pull-downs (Streptavidin PD) were analyzed with the antibodies indicated.