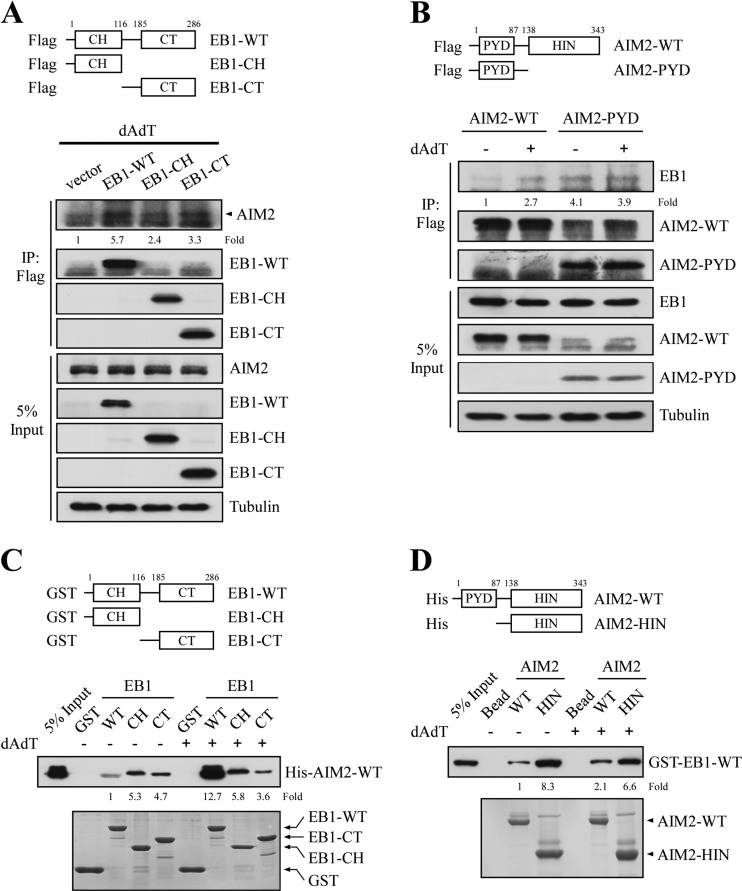
Fig. 4.
EB1 directly interacts with AIM2 in vitro. A, NPC TW02 cells were transfected with empty vector or vectors encoding Flag-EB1-WT, Flag-EB1-CH, or Flag-EB1-CT. After 48 h, the cells were treated with poly (dA:dT) for 2 h and incubated for 4 h, and then harvested for immunoprecipitation using an anti-Flag matrix. The levels of AIM2 were assessed by Western blotting after immunoprecipitation. The fold-change numbers were obtained from three independent experiments. B, HK1 cells stably expressing Flag-AIM2-WT or Flag-AIM2-PYD (introduced by lentiviral infection) were treated with or without poly (dA:dT) and subjected to immunoprecipitation using an anti-Flag matrix. The levels of EB1 were assessed by Western blotting. C, Purified GST, GST-EB1-WT, GST-EB1-CH, and GST-EB1-CT fusion proteins were immobilized on glutathione agarose, and then separately incubated with purified His-AIM2-WT fusion proteins for 24 h. The bound proteins were washed, and then analyzed by Western blotting with an anti-His antibody. The fold-change numbers were derived from three independent experiments. The amounts of GST fusion proteins were assessed by Coomassie blue staining. D, Purified His-AIM2-WT or His-AIM2-HIN were immobilized on Ni-charged agarose, and then incubated with purified GST-EB1-WT for 24 h. The bound proteins were washed, and then analyzed by Western blotting with an anti-GST antibody. The fold-change numbers were obtained from three independent experiments. The amount of His fusion proteins were assessed by Coomassie blue staining.