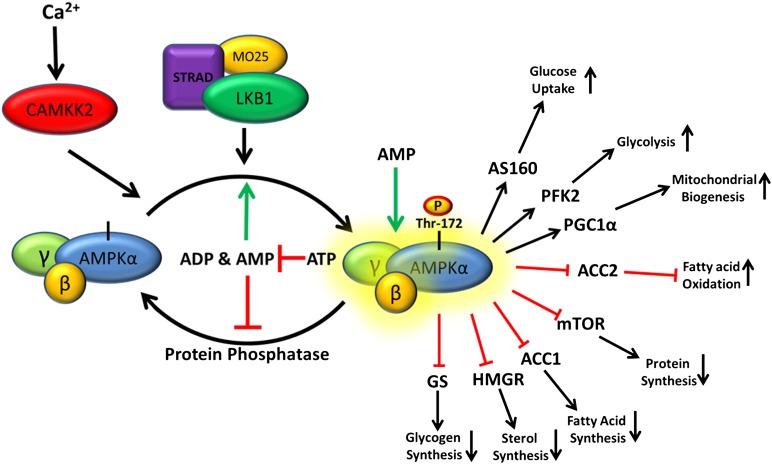
Fig. 1.
Current model for AMPK activation. Constitutively active LKB1-STRAD-MO25 complex continuously phosphorylates AMPK, which in the absence of low energy signals is rapidly dephosphorylated by protein phosphatases. In times of energy stress, when the rate of ATP consumption exceeds the rate of production, the ADP:ATP ratio increases. Through the action of adenylate kinase, increases in ADP concentrations are associated with concomitant increases in AMP concentrations, and the intracellular AMP:ATP ratio also increases. This energy stress favors binding of ADP/AMP over ATP to the γ-subunit, leading to a conformational change that increases LKB1-STRAD-MO25-dependent phosphorylation and decreases susceptibility to protein phosphatase-dependent dephosphorylation. The combined effect of increased AMPKα phosphorylation and AMP allosteric activation can yield > 1,000-fold increase in activity. Activated AMPK then mediates its control on metabolism by phosphorylation of many downstream targets, resulting in restored energy homeostasis.