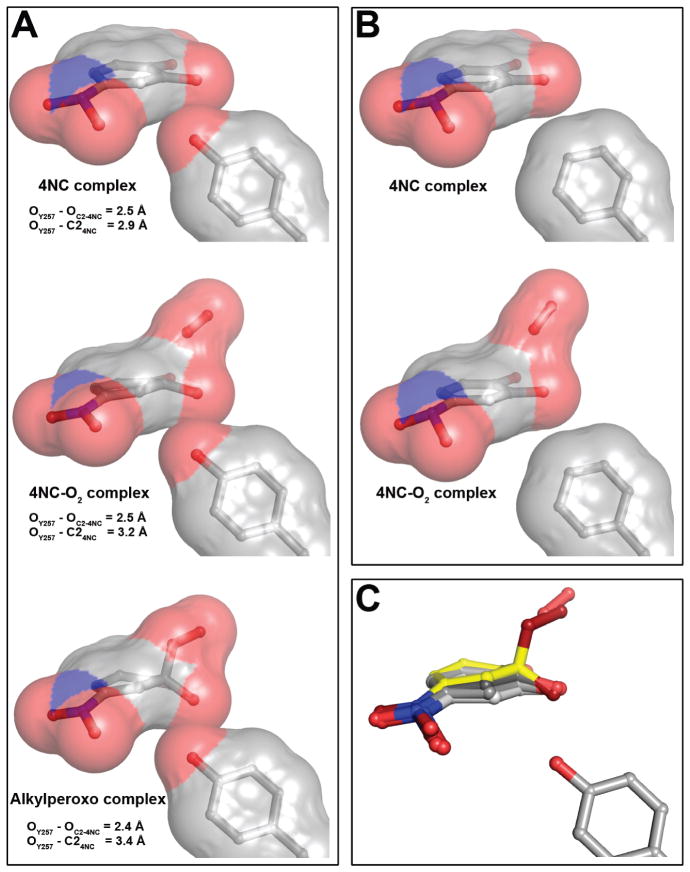
Figure 8.
Representative steric interactions between ring carbon atoms of 4NC-derived intermediates and 2nd sphere residue in position 257 in the FeHPCD and Y257F enzymes. (A) The models and van der Waals interactions (surface) for the FeHPCD enzyme: 4NC complex (PDB 4GHH, subunit C), [4NC-semiquinone]-O2•− (PDB 2IGA, subunit C) and alkylperoxo intermediate (PDB 2IGA, subunit D). (B) The models and van der Waals interactions (surface) for the Y257F variant: 4NC complex (PDB 4GHE, subunit C) and 4NC-oxy (PDB 4GHF, subunit C). Atom color code: gray, carbon; blue, nitrogen; red, oxygen. (C) Overlay of 4NC-derived intermediate structures, illustrating the gradient of localized distortion of the 4NC ring at C2. Atom color code: light gray, carbon (planar 4NC from PDB 4GHE, subunit C); gray, carbon (strained 4NC from PDB 4GHH, subunit C); dark gray, carbon (4NC-semiquinone from PDB 2IGA, subunit C); yellow, carbon (alkylperoxo intermediate from PDB 2IGA, subunit D); blue, nitrogen; dark red, oxygen (alkylperoxo intermediate); red, oxygen.