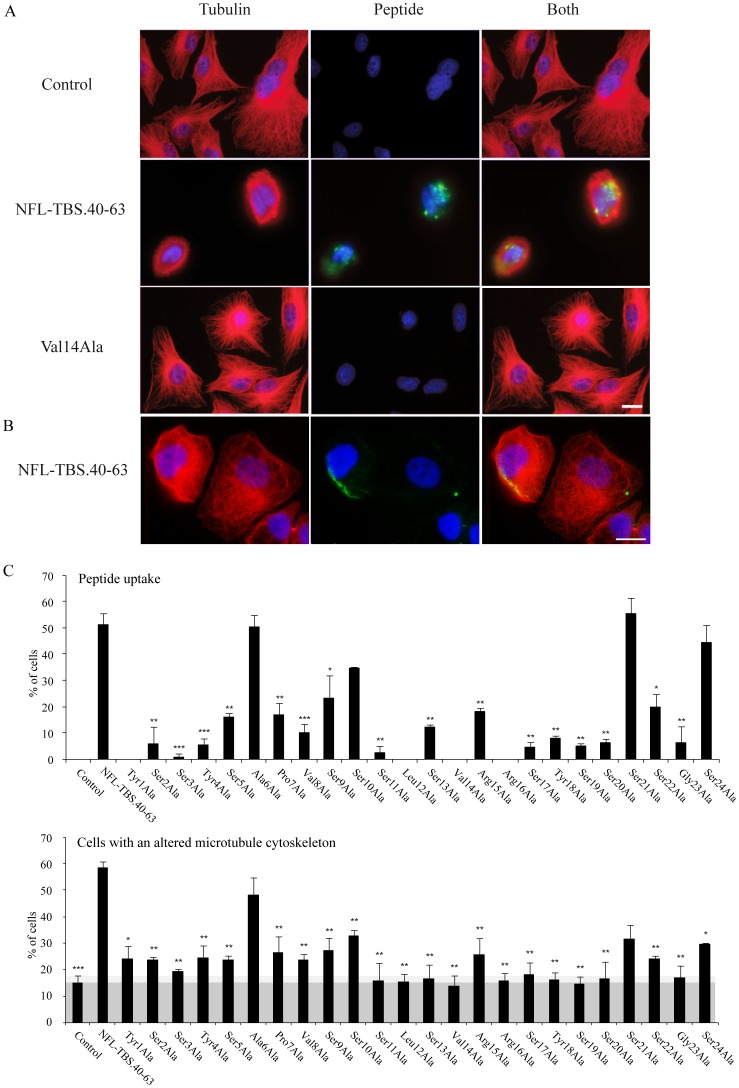
Figure 1. Internalization and effects on glioblastoma T98G cells of the wild type peptide NFL-TBS.40-63 and peptides from the alanine scan.
(A): Human glioblastoma T98G cells were incubated in the presence of different peptides at 10 µM during 6 hours. MTs were detected by immunostaining using an anti-tubulin antibody (red), and biotinylated peptides were detected using Alexa-labeled avidin (green). While the original NFL-TBS.40-63 peptide is able to penetrate in these cells, the replacement of valine-14 by alanine typically abolished this property. White bars, 20 µm. (B) Human adrenal carcinoma SW13 cells were incubated in the presence of NFL-TBS.40-63 peptide (10 µM, 6 hours). MTs appear in red, peptide in green, nuclei in blue. White bars, 20 µm. (C): We quantified by microscopy the percentage of T98G cells containing the peptide and those with a destroyed MT network. Experiments were triplicated and a minimum of 200 cells was examined in each experiment. Data are presented as mean and S.E.M. (bars). Asterisks indicate significant level versus control: * p<0.05; ** p<0.005; *** p<0.001. As glioblastoma cells are known to have multiple mutations and abnormalities, the shaded area corresponds to the percentage of cells where MTs are disorganized even in the absence of peptide.