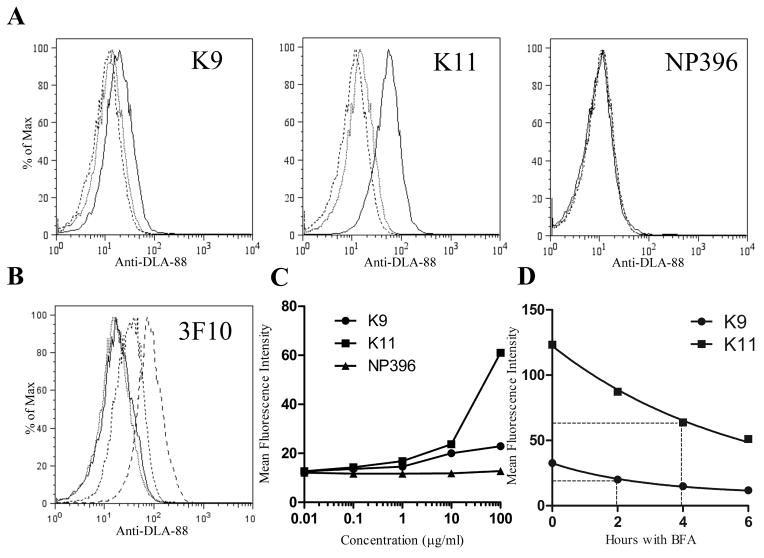
Fig. 2.
DLA-88*50801 is stabilized on the surface of BARC3 cells by the addition of DLA-88*50801 binding peptides to the culture medium. (A) The canine self peptides K9 and K11, but not the H2-Db-binding epitope NP396, increase surface expression of DLA-88*50801 in a dose-dependent manner. BARC 3 cells were cultured in the presence of 100 (solid line), 1 (dotted line), or 0.01 (dashed line) μg/ml of the indicated peptide; for clarity, data from 10 and 0.1 μg/ml peptide cultures are not shown. (B) Staining of K9- and K11-pulsed BARC3 cells with the anti-class I Ab 3F10 shows an increase in surface DLA-88*50801 expression that is similar to that observed using H58A. BARC3 cells were incubated with 100 μg/ml of NP396 (dotted line), K9 (dashed line), K11 (long dashed line), or no peptide (solid line). (C) Graphical representation of data shown in (A). (D) The K9 and K11 peptides dissociate from peptide-MHC complexes at different rates. BARC3 cells were cold-cultured overnight in the presence of 100 μg/ml of the indicated peptide, succeeded by an additional 5 hours at 37°C to remove unstable complexes. After washing to remove unbound peptide, cells were incubated at 37°C with BFA and harvested at the indicated time points. The dissociation half-life (t1/2 – shown by dotted lines) was calculated by non-linear regression, using a one phase exponential decay model for curve fitting. In the first experiment, the t1/2 for K9 was 1.9 h (95% CI 1.2 – 3.2 h), and 6.0 h for K11 (95% CI 3.6 – 18.9 h). In the second experiment (shown in graph), the t1/2 for K9 was 2.0 h (95% CI 1.7 – 2.4 h), and 4.0 h for K11 (95% CI 3.3 – 5.0 h). In this figure, data are representative of two [(B), (D)] or three [(A)] independent experiments.