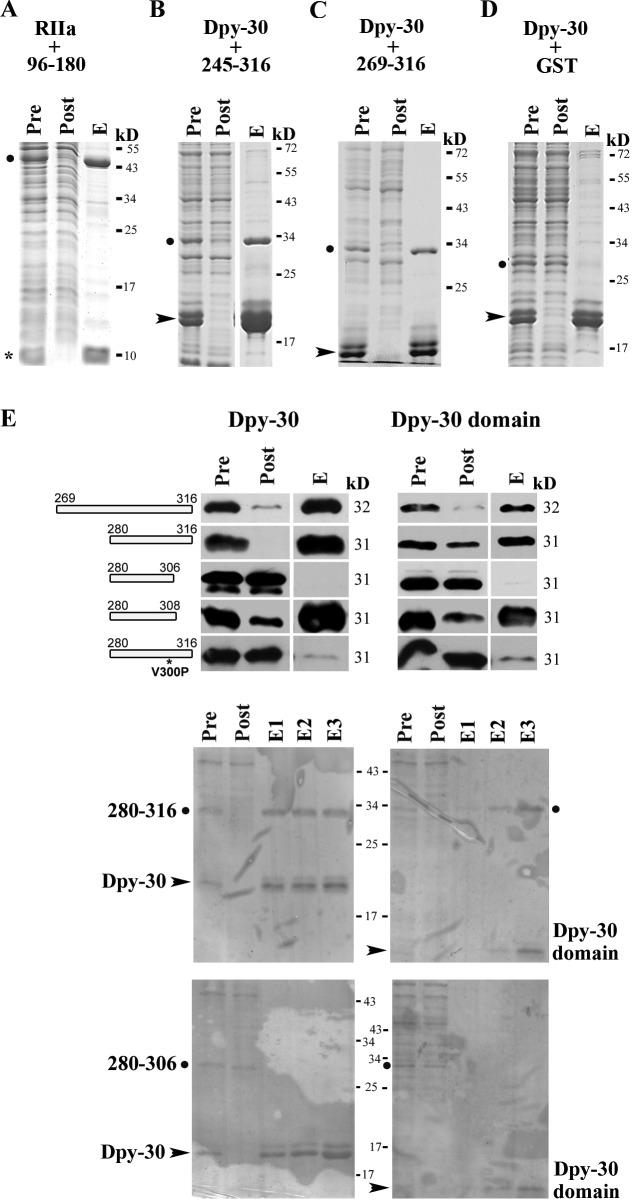
Figure 7.
Two sites in RSP3 bind to the RIIa and the Dpy-30 domain. His-tagged RIIa from RSP7 (asterisk) or His-tagged Dpy-30 protein (arrowhead) were coexpressed with a GST-tagged RSP3 peptide (dot) in bacteria. The Ni-NTA pulldown from the extracts was analyzed by Coomassie-stained gel (A–D) or GST Western blots (E). (A) The positive control, the copurification of the RIIa domain, and RSP396–180 that contains the AHR (Gaillard et al., 2001). (B and C) The copurification of His-tagged human Dpy-30 protein and GST-tagged RSP3245–316 or RSP3269–316 (dot). (D) The negative control. GST alone did not interact with Dpy-30 protein. (E) The smallest region that binds the Dpy-30 domain is aa 280–308 in RSP3. The interaction is perturbed by V300P mutation. His-tagged full-length Dpy-30 protein or the Dpy-30 domain was only coexpressed with GST-tagged RSP3 peptides as indicated. The irregular patterns in the Ponceau-stained membranes for the demonstration of protein loads (middle and bottom) were from plastic wraps and staining solution. Pre, the bacterial extract; Post, the flow-through from Ni-NTA matrix; and E, one of the eluates that contained the most Dpy-30 (see Materials and methods). Dpy-30 often migrated as double bands because of the susceptibility of its C-terminal end to proteolysis.