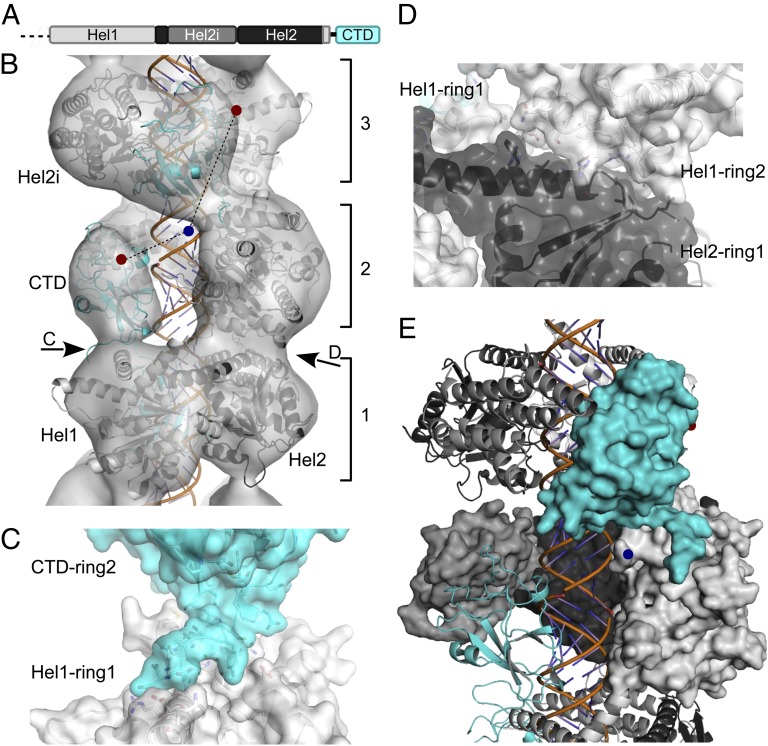
Fig. 2.
Atomic model of the MDA5-dsRNA filament with ATP-γ-S present. (A) Domain organization of ΔCARD-MDA5. (B) Atomic homology models fitted into the ΔCARD-MDA5 reconstruction. The C terminus of Hel1 (blue dot) may connect to CTD N termini in the same or adjacent rings (red dots), 20 Å and 38 Å away, respectively. (C and D) Ring contacts are formed by a Hel1–CTD interface (C) and a Hel1–Hel2 interface (with minor Hel1–Hel1 contacts) (D). Note that the C-terminal sequence of the CTD was modeled in an extended conformation (Materials and Methods). (E) The ring-invading Hel1-CTD model results in an intermolecular interface between the Hel2i domain (medium gray) and the CTD (cyan). One ΔCARD-MDA5 molecule is shown as a surface; adjacent molecules are shown as cartoons.