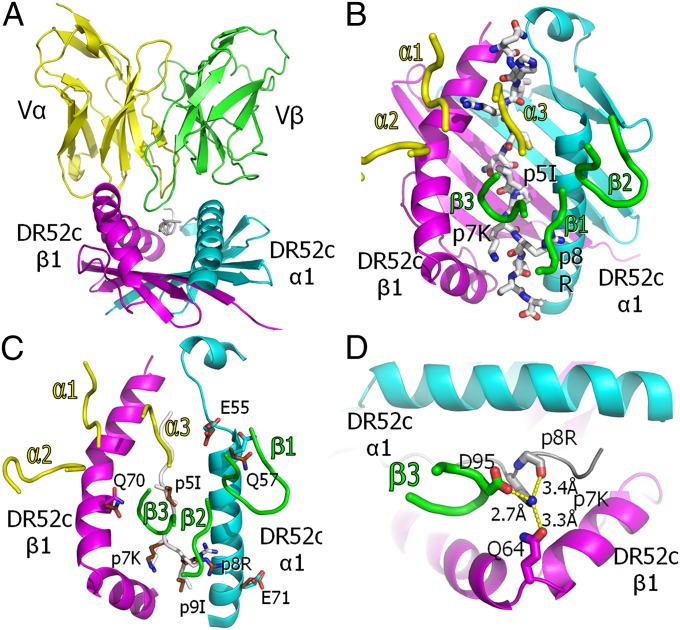
Fig. 4.
Conventional orientation of the ANi2.3 TCR on pHIR–DR52c and Vβ CDR3 coordination of the highly selected p7K. (A) Ribbon representations of the Vα (yellow) and Vβ (green) domains of the ANi2.3 TCR bound to the DR52c–pHIR complex: DR52c α1, cyan; β1, magenta; pHIR, white. The view is from the peptide C terminus down the peptide binding groove. (B) Ribbon representations of DR52c α1 (cyan) and β1 (magenta) domains, and a wire-frame representation of the pHIR peptide (O, red; N, blue; C, white). The ANi2.3 TCR CDR loops are presented as tubes (Vα, yellow; Vβ, green). (C) DR52c and the ANi2.3 CDR3 loops are shown as in B, and the pHIR backbone is shown as a white tube. Side chains of the eight amino acids of the ligand that change their conformation upon ANi2.3 binding are shown in wire-frame representation (O, red; N, blue; α1: C, cyan; β1: C, magenta; peptide: C, white). Superimposed are the same side chains in the unbound DR52c–pHIR structure (O, red; N, blue; C, brown). (D) Top view of salt bridge/H bond coordination of the εNH2 of pHIR p7K with the carboxylate of ANi2.3 VβCR3 95D, the carbonyl oxygen of DR52c β1 Q64, and the backbone oxygen of p8R.