Fig. 5.
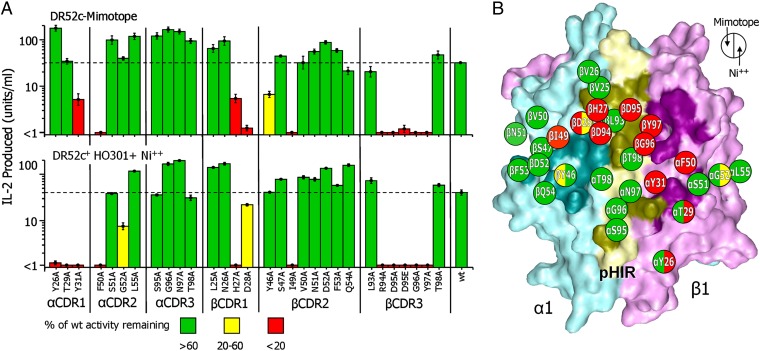
Similar pattern of ANi2.3 TCR interaction with the pHIR mimotope and Ni++. (A) The bar graph shows the sensitivity of the response of ANi2.3 to mutation of 29 amino acids of its six CDR3 loops to alanine or mutation of Vβ D95 to E. T cells bearing the wild-type or each mutant TCR were tested for response to DT40 chicken cells bearing DR52c covalently attached to the pHIR mimotope (Upper) or to HO301 DR52c+ lymphoblastoid cells plus 100 µM Ni++ (Lower) as measured by IL-2 secretion. The average ± SEM of three experiments is shown. The bars for responses that were less than 20% of the wild-type response are colored red, yellow (between 20% and 60%), and green (greater than 60%). (B) The solvent-accessible surface of the DR52 α1 (cyan) and β1 (magenta) and pHIR (yellow) is shown with the footprint of the ANi2.3 TCR in darker versions of the same colors. The 29 mutated residues of the ANi2.3 TCR are schematically mapped onto the pHIR–DR52 surface with their side-chain positions labeled within a circle. The left half of each circle is colored the same as the bars in A, Upper and the right half is colored the same as the bars in A, Lower.