Abstract
Individual differences in cognitive function are highly heritable and most likely driven by multiple genes of small effect. Well-characterized common functional polymorphisms in the genes MAOA, COMT, and 5HTTLPR each have predictable effects on the availability of the monoamine neurotransmitters dopamine, noradrenaline, and serotonin. We hypothesized that 5HTTLPR genotype would show little association with prefrontal cognitive performance, but that COMT and MAOA would have interacting effects on cognition through their shared influence on prefrontal catecholamine availability. We assessed the individual and epistatic effects of functional polymorphisms in COMT, MAOA, and 5HTTLPR on children's prefrontal cognitive function in nearly 6,000 children from the population-based Avon Longitudinal Study of Parents and Children (ALSPAC). Neither MAOA nor 5HTTLPR polymorphisms showed significant effects on cognitive function. In boys but not girls, there was a modest but statistically significant interaction between MAOA and COMT genotypes such that increased prefrontal catecholamine availability was associated with better working memory. These results suggest that assessment of multiple genes within functionally related systems may improve our understanding of the genetic basis of cognition. © 2010 Wiley-Liss, Inc.
Keywords: genetics, cognition, serotonin, dopamine
INTRODUCTION
Experimental manipulation of human and primate neurotransmission has provided insight into the distinct roles that the monoamine neurotransmitters serotonin (5-HT), dopamine (DA), and noradrenaline (NA) play in prefrontal cognition [Clarke et al., 2004; Chamberlain et al., 2006; Ramos and Arnsten, 2007[. For example, while prefrontal DA is central to working memory [Brozoski et al., 1979[, experimental lowering of serotonin has little effect on prefrontally mediated tasks, but does impair learning [Park et al., 1994; Clark et al., 2005; Talbot et al., 2006[. Differentiating the cognitive effects of NA from its precursor, DA, can be difficult, but drugs which specifically mimic NA actions at a2 adrenergic receptors restore prefrontal cortex (PFC) function in catecholamine-depleted monkeys and rats [Arnsten and Goldman-Rakic, 1985; Carlson et al., 1992[ and improve executive functions in patients [Mair and McEntee, 1986; Fields et al., 1988; Taylor and Russo, 2001[ and healthy volunteers [Jakala et al., 1999[.
Two major routes for the deactivation of monoamine neurotransmitters are reuptake by transporter molecules in the presynaptic cell membrane, and enzymatic degradation within the neuron or synapse. Both serotonin and NA transporters are abundant in PFC [Mantere et al., 2002; Miner et al., 2003[ but in primates the DA transporter is located primarily extrasynaptically [Lewis et al., 2001[, resulting in a more prominent role for enzymatic degradation of synaptic DA. It is estimated that catechol-O-methyltransferase (COMT), one such enzyme [Karoum et al., 1994; Gogos et al., 1998[, may be responsible for half of prefrontal DA decline [Yavich et al., 2007[. In contrast, COMT appears to have relatively little effect on NA in frontal cortex: COMT knockout mice show normal prefrontal NA levels and administration of the COMT-inhibitor tolcapone increases the release of DA, but not NA, in rat PFC [Gogos et al., 1998; Tunbridge et al., 2004[. A second set of enzymes, the monoamine oxidases, catalyze the oxidation of monoamine neurotransmitters, including serotonin, DA, and NA from their location in the mitochondrial outer membrane [Green and Youdim, 1975[. The A isoform (MAOA) is more abundant in catecholaminergic neurons, while MAOB is more abundant in serotonergic neurons [Levitt et al., 1982; Westlund et al., 1988; Willoughby et al., 1988; Saura et al., 1992[.
Individual differences in cognitive function are highly heritable [Devlin et al., 1997[, and this may be driven partly by genetic variants, which directly affect prefrontal neurotransmitter availability. Functional polymorphisms in COMT, MAOA, and the serotonin transporter-linked polymorphic region (5HTTLPR) affect the level and/or activity of their products in defined manners, summarized in Table I. Cognitive effects of these functional polymorphisms have been studied to varying extents. Egan et al. [2001[ reported that the COMT Val allele was associated with worse performance on a measure of executive function, the Wisconsin Card Sort Test (WCST). While meta-analysis of similar studies shows little consistent cognitive effect [Barnett et al., 2008[, consistent differences are seen in prefrontal activation during working memory tasks, with greater activation among Val allele carriers [Egan et al., 2001; Mier et al., 2010[. In contrast, relatively little is known about the effects of the MAOA and 5HTTLPR polymorphisms on cognition. MAOA genotype has been reported to significantly affect IQ among healthy Chinese women [Yu et al., 2005[ and children with autism [Yirmiya et al., 2002; Cohen et al., 2003[ but with conflicting directions of effect. Reports regarding cognitive effects of the 5HTTLPR polymorphism have been predominantly negative for standard neuropsychological measures such as IQ, response inhibition, and sustained attention [Fallgatter et al., 1999; Clark et al., 2005; Payton et al., 2005; Roiser et al., 2007; Strobel et al., 2007; da Rocha et al., 2008; Borg et al., 2009[, though there are now multiple reports of associations with risk-taking, decision-making, and attentional biases [Beevers et al., 2007; Homberg et al., 2008; da Rocha et al., 2008; Crisan et al., 2009; Firk and Markus, 2009; Fox et al., 2009; Roiser et al., 2009[.
TABLE I.
Genetic Polymorphisms Examined in the Avon Longitudinal Study of Parents and Children (ALSPAC)
| Gene | Effects on 5-HT | Effects on NA | Effects on DA | Polymorphism | Functional effects | Predicted effects on prefrontal cognition |
|---|---|---|---|---|---|---|
| Monoamine oxidase A; (MAOA) | Yes | Yes | Yes | 30-bp VNTR in promoter | 3-repeat allele results in approximately fivefold lower transcription than 3.5- or 4-repeat alleles [Sabol et al., 1998[ | Strong |
| Catechol-O-methyltransferase (COMT) | No | Minor | Yes | SNP in exon 4 (Val158Met; rs4680) | rs4680 alters COMT enzyme thermostability, resulting in 40% lower activity in Met allele carriers at body temperature [Chen et al., 2004[ | Strong |
| Serotonin transporter (SLC6A4) linked polymorphic region (5HTTLPR) | Yes | No | No | 43-bp VNTR and SNP (rs25531) in promoter | Threefold reduction in transporter activity in the short (S) relative to the long (L) allele of the VNTR [Heils et al., 1996; Lesch et al., 1996[. SNP rs25531, immediately upstream of the VNTR [Kraft et al., 2005; Wendland et al., 2006[ reduces expression of L allele carriers back to levels equivalent to S carriers [Hu et al., 2006[ | Weak |
Despite a number of studies in these and other genes, few associations between genetic polymorphisms and cognition have been convincingly replicated. In the largest candidate gene study of cognition to date, we previously reported small but significant sex-specific effects of Val158Met on cognitive performance at ages 8 and 10 in a population-based sample of more than 5,000 children from the Avon Longitudinal Study of Parents and Children (ALSPAC) [Barnett et al., 2007[. Among boys, but not girls, Met allele carriers showed better verbal IQ and verbal response inhibition at age 8, and better working memory at age 10. Here we report the effects in the same sample of functional polymorphisms in MAOA and 5HTTLPR. We also assess evidence for epistasis between the MAOA, 5HTTLPR, and COMT polymorphisms, in an attempt to broaden the candidate gene approach to include assessment of functionally interactive gene systems, here represented by the overlapping effects of these SNPs on synaptic neurotransmitter availability. We hypothesized that genes that exclusively affect serotonergic function (5HTTLPR) would show little effect on these prefrontally driven cognitive tasks, but that main genetic effects, and potential epistasis, would be demonstrated for variants affecting dopaminergic or noradrenergic availability (COMT, MAOA; see Table I and Fig. 1).
FIG 1.
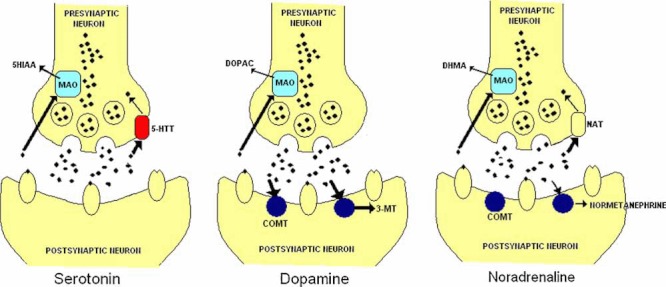
Schematic diagram of major routes of inactivation of the monoamine neurotransmitters serotonin, dopamine, and NA in prefrontal cortical synapses. The enzyme monoamine oxidase, located in the mitochondrial cell wall degrades 5-HT, DA, and NA into the compounds 5HIAA, DOPAC, and DHMA. A second enzyme, catechol-O-methyltransferase (COMT) degrades dopamine and, to a lesser extent, NA, forming the products 3-methoxytyramine (3-MT) and normetanephrine. Transporter proteins located in the cell membrane allow reuptake of serotonin and NA into the presynaptic neuron, but dopamine transporters are predominantly located extrasynaptically in primate prefrontal cortex [Lewis et al., 2001[, increasing the importance of enzymatic degradation of DA.
MATERIALS AND METHODS
Sample
ALSPAC is a general population cohort based in southwest England. The cohort comprised 14,062 live births from 14,541 enrolled pregnancies which were due to give birth between 01 March 1991 and 31 December 1992 [Golding et al., 2001[. This study is based on cognitive assessments completed at ages 8 and 10. To increase sample homogeneity, only children of Caucasian ethnicity (95% of the cohort) were included in these analyses. Parents gave informed consent at enrolment and ethical approval was obtained from the ALSPAC and Local Research Ethics Committees.
Cognitive Assessments
As in our previous study [Barnett et al., 2007[, we examined associations between genotype and prefrontal-dependent tests of cognition completed at ages 8 (mean 8 years 8 months, SD 3.1 months) and 10 (mean 10 years 8 months, SD 3.0 months). At age 8, verbal and performance IQ was assessed using alternate items from the Wechsler Intelligence Scale for Children (WISC) 3rd UK edition [Wechsler et al., 1992[ and verbal inhibition was assessed with the Opposite Worlds task from the Test of Everyday Attention for Children battery [Manly et al., 2001[, which required the child to read aloud a string of the digits 1 and 2 and responded in the “opposite” manner (i.e., saying “one” for the digit 2). Working memory was assessed at age 10 using the Count Span task [Case et al., 1982[, which required the child to count out loud the number of red dots presented on a screen. After multiple screens the child was asked how many dots were on each screen within that set, and the number of trials correct (maximum 42) was reported.
Genotyping
DNA, obtained from blood and mouthwash samples, was extracted and processed [Jones et al., 2000[, and COMT Val/Met [Barnett et al., 2007[, and 5HTTLPR [Araya et al., 2009[ genotyping were conducted as previously described. For MAOA, because of the high GC content around the VNTR, amplifications were performed using Invitrogen's PlatinumTaq and PCRX Enhancer System kits, according to the manufacturer's protocol (Invitrogen, Carlsbad, CA), with 5 µM of each primer and 25 mM dNTPs in a total reaction volume of 25 µl. Amplifications were performed on a Perkin Elmer 9700 thermocycler (Applied Biosystems, Foster City, CA) with one cycle at 96°C for 10 min followed by 30 cycles of 94°C for 15 sec, 60°C for 15 sec, 72°C for 30 sec, and a final 3-min extension at 72°C. The forward primer was labeled with the fluorescent dye 6-FAM, and amplicons were visualized on an ABI 3100 capillary sequencer. Allele sizes (allele 2, 233 bp; allele 3, 263 bp; allele 3.5, 278 bp; allele 4, 293 bp; and allele 5, 323 bp) were determined using Genotyper 2.5 (Applied Biosystems). MAOA 3, 3.5, and 4-repeat allele frequencies in girls were consistent with Hardy–Weinberg equilibrium (χ2 = 1.27, DF 3, P = 0.74).
Analysis
Individuals were classified into high, medium, or low activity groups with respect to the functional effects of the polymorphism in each of the three genes. In each case, “high activity” groups comprised the alleles leading to fastest clearance of the synaptic neurotransmitter and “low activity” the alleles leading to slowest clearance. For 5HTTLPR, low activity individuals were those with SS, SLG, or LGLG genotypes, medium activity were SLA or LGLA genotypes, and LALA genotypes were denoted high activity [Hu et al., 2006[ since this genotype results in increased serotonin transporter protein and hence faster 5-HT uptake [Lesch et al., 1996[. For COMT, Met/Met individuals were considered low, Val/Met individuals medium, and Val/Val individuals high activity, because Val allele carriers show higher COMT enzyme activity, and hence faster degradation of catecholamines at body temperature [Lachman et al., 1996; Chen et al., 2004[. For MAOA we excluded genotypes involving the rare 2- or 5-repeat alleles, whose functional effects are not yet clear [Sabol et al., 1998; Deckert et al., 1999[. Since MAOA is located on the X chromosome, boys carry only one allele. Boys with the 3-repeat allele were denoted low MAOA activity and those with 3.5- or 4-repeat alleles as high activity, since they show higher transcriptional efficiency and hence result in higher levels of synaptic MAOA [Sabol et al., 1998; Deckert et al., 1999[. For girls, 3/3 homozygotes were denoted low activity, 3/3.5 or 3/4 heterozygotes medium activity, and genotypes comprising two copies of 3.5- or 4-repeat alleles were denoted high activity.
Main effects of genes on cognition were assessed by comparing cognitive scores between high, medium, and low activity groups using one-way ANOVA for the 5HTTLPR, and for MAOA in girls only. For boys, we compared cognitive scores in high versus low activity groups using t-tests. Main effects of COMT in this sample have been previously reported [Barnett et al., 2007[ and hence were not assessed here.
Two possible sources of epistatic effects on cognition were considered: a serotonergic interaction between 5HTTLPR and MAOA genotypes, and a catecholaminergic one between COMT and MAOA genotypes. We assessed evidence for these interactions using general linear models with each cognitive measure as an outcome variables predicted by two genetic main effects and a gene–gene interaction term. As before, all genotypes were coded as low, medium, or high activity. These analyses were conducted separately in boys and girls because MAOA is located on the X chromosome, and because COMT is known to have sexually dimorphic effects on a range of functional and neuropsychiatric phenotypes [Harrison and Tunbridge, 2008[, including cognitive function in this sample [Barnett et al., 2007[.
RESULTS
Data Availability
The number of children included in each analysis varied by the availability of cognitive data and genotypes: cognitive data were available on around two-thirds of those for whom DNA is available. As previously reported [Araya et al., 2009[, after stringent quality control measures, fewer children were available for inclusion in analyses involving 5HTTLPR genotype. There were no cognitive differences between children for whom 5HTTLPR, MAOA, or COMT genotypes were or were not available (all P > 0.05). Allele frequencies were, for 5HTTLPR alleles S = 41.4%, LA = 51.5%, LG = 7.1%, and for MAOA, 2-repeat = 0.2%, 3-repeat = 34.0%, 3.5-repeat = 2.0%, 4-repeat = 62.4%, 5-repeat = 1.5%. COMT allele frequencies, as previously reported, were Val = 48.6%, Met = 51.4%. The number of children considered as low, medium, and high activity genotypes for whom at least one cognitive measure was available are shown in Table II.
TABLE II.
Sample Sizes of High, Medium, and Low Activity MAOA, 5HTTLPR, and COMT Genotype Groups in ALSPAC Cognitive Study
| High activity | Medium activity | Low activity | ||||
|---|---|---|---|---|---|---|
| Total (n) | Genotypes | n | Genotypes | n | Genotypes | n |
| MAOA girls | ||||||
| 2,984 | 3.5/3.5; 3.5/4; 4/4 | 1,245 | 3/3.5; 3/4 | 1,380 | 3/3 | 359 |
| MAOA boys | ||||||
| 3,066 | 3.5; 4 | 2,037 | NA | 0 | 3 | 1,029 |
| 5HTTLPR | ||||||
| 4,579 | LALA | 1,153 | SLA; LGLA | 2,346 | SS; SLG; LGLG | 1,080 |
| COMT | ||||||
| 5,909 | Val/Val | 1,396 | Val/Met | 2,921 | Met/Met | 1,592 |
In each case “high activity” refers to the allele resulting in faster clearance of synaptic neurotransmitter.
Main Effects of MAOA
Effects of MAOA on cognitive measures were assessed separately in boys and girls. t-Tests between high (minimum n = 1,685) and low (minimum n = 854) activity MAOA groups in boys showed no effects on any cognitive measure. Similarly in girls, one-way ANOVA between low, medium, and high activity MAOA groups (minimum sample sizes, respectively, 290, 1,170, 1,024) revealed no cognitive differences (Table III).
TABLE III.
Main Effects of MAOA and 5HTTLPR Genotype on Cognitive Function in ALSPAC
| Measure | Total (n) | Low | Medium | High | F | DF | P-value |
|---|---|---|---|---|---|---|---|
| 5HTTLPR | |||||||
| Verbal IQ | 4,174 | 107.5 (16.6) | 107.8 (16.7) | 108.5 (16.2) | 1.20 | 2,4171 | 0.30 |
| Performance IQ | 3,882 | 100.4 (16.6) | 100.4 (16.8) | 100.5 (16.1) | 0.05 | 2,3879 | 0.95 |
| Verbal inhibition | 4,109 | 0.244 (0.026) | 0.244 (0.025) | 0.245 (0.025) | 0.55 | 2,4106 | 0.58 |
| Working memory | 3,880 | 18.7 (7.49) | 18.5 (7.75) | 19.2 (7.58) | 3.13 | 2,3877 | 0.04 |
| MAOA boys | |||||||
| Verbal IQ | 2,717 | 108.2 (17.2) | — | 108.1 (17.2) | 0 | 1,2715 | 0.99 |
| Performance IQ | 2,555 | 99.6 (17.0) | — | 99.5 (17.2) | 0.01 | 1,2553 | 0.93 |
| Verbal inhibition | 2,678 | 0.242 (0.025) | — | 0.242 (0.025) | 0.38 | 1,2676 | 0.54 |
| Working memory | 2,537 | 18.4 (8.06) | — | 18.3 (7.87) | 0.25 | 1,2535 | 0.62 |
| MAOA girls | |||||||
| Verbal IQ | 2,673 | 106.8 (15.4) | 107.3 (15.8) | 106.9 (15.9) | 0.19 | 2,2670 | 0.82 |
| Performance IQ | 2,484 | 100.9 (16.1) | 100.1 (16.2) | 101.1 (16.5) | 1.17 | 2,2481 | 0.31 |
| Verbal inhibition | 2,643 | 0.247 (0.025) | 0.246 (0.025) | 0.246 (0.025) | 0.25 | 2,2640 | 0.78 |
| Working memory | 2,565 | 18.8 (7.62) | 18.8 (7.13) | 18.8 (7.55) | 0.04 | 2,2562 | 0.96 |
Main Effects of 5HTTLPR
Groups defined by low, medium, and high serotonin transporter promoter activity were compared on all cognitive measures using one-way ANOVA. There were no differences between groups on measures of IQ or verbal inhibition (see Table III). Nominal differences were seen between groups on working memory score (F = 3.13, DF 2,3877, P = 0.04, uncorrected) such that better performance was seen in the high than medium activity group (Tukey's post hoc P = 0.03). One-way ANOVA revealed no cognitive differences between the three genotypes (SS, SLG, and LGLG) within the low activity group.
Gene × Gene Interactions
Models comprising main effects of COMT genotype and MAOA genotype, and a COMT × MAOA interaction term, were fitted separately for girls and boys. In boys, there was evidence of interaction between COMT and MAOA only for working memory score (model fit F = 3.54, DF 5,2324, P = 0.003; main effect of COMT F = 6.89, DF 1,2324, P = 0.001; main effect of MAOA F = 0.68, DF 1,2324, P = 0.41; COMT × MAOA interaction term F = 4.17; DF 2,2324, P = 0.016; see Fig. 2). Consistent with our previous report [Barnett et al., 2007[, these models showed main effects of COMT genotype on verbal IQ, verbal inhibition, and working memory, in all cases such that Met alleles were advantageous. In contrast, in girls there were no significant main effects nor interaction terms for any cognitive outcome.
FIG 2.
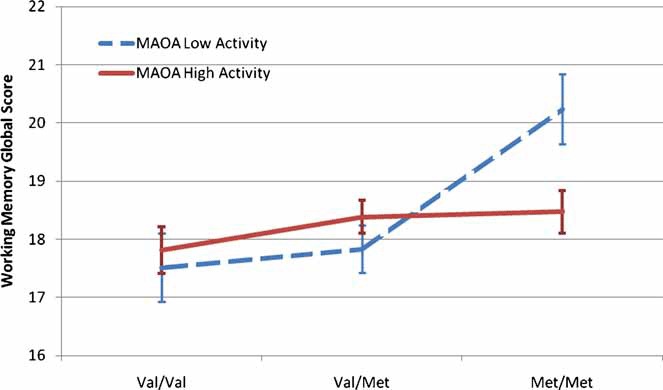
Working memory global score (mean, SE) by COMT and MAOA genotype in 2,324 boys at age 10 years. COMT × MAOA interaction term: F = 4.17; DF 2,2324, P = 0.016.
Models comprising main effects of MAOA and 5HTTLPR, and a MAOA × 5HTTLPR interaction term, did not show significant fit for any cognitive measure in either boys or girls.
DISCUSSION
In a large, population-based sample, we assessed the cognitive effects of functional polymorphisms in three genes which have predictable and partially overlapping effects on serotonin, DA, and NA availability in the PFC. We found no evidence for effects of serotonin availability on measures of IQ, verbal inhibition, or working memory, and no evidence for cognitive effects of interactions between the 5HTTLPR and MAOA. Contrary to our hypotheses, we found no evidence for main effects of MAOA variation on cognitive functions, but there were apparent interactions between the COMT and MAOA genes in their effects on working memory in boys. Here we consider possible explanations for this pattern of limited and sex-specific cognitive genetic effects.
The lack of serotonergic effects on prefrontal cognition reported here fit with the majority of previous evidence, where serotonergic manipulations, including variation in 5HTTLPR have been previously reported to affect aspects of decision-making and attentional control [Beevers et al., 2007; Homberg et al., 2008; Fox et al., 2009; Roiser et al., 2009[ but not IQ or response inhibition [Fallgatter et al., 1999; Clark et al., 2005; Payton et al., 2005; da Rocha et al., 2008[. Unlike previous studies, the present sample was sufficiently large to detect even very small associations between genotype and cognition (93% power to detect the effect of a locus explaining just 0.2% of variance in the sample, given n = 6,000, alpha = 0.05, and assuming no dominance). We can therefore definitively say that there is no evidence for cognitive effects of 5HTTLPR on these measures of working memory, response inhibition, or IQ in healthy children. It remains plausible, however, that genetic effects on cognition are seen in circumstances in which the serotonergic status quo has been disrupted, for example, by stress, tryptophan depletion [Roiser et al., 2007; Firk and Markus, 2009[ or in neuropsychiatric disorders [Beevers et al., 2007; da Rocha et al., 2008[. Some apparently conflicting results may therefore have arisen because cognitive differences between genotypes appear exaggerated in circumstances of serotonergic perturbation, where normal mechanisms of compensation for genetic variation are disturbed.
It is notable that no genetic associations were observed on the Opposite Worlds verbal inhibition task, other than the previously reported main effects of COMT genotype [Barnett et al., 2007[. While previous reports of associations between response inhibition tasks and 5HTTLPR have been negative [Fallgatter et al., 1999; Clark et al., 2005[, a recent neuroimaging study found both main effects of 5HTTLPR and MAOA polymorphisms, and interactions between the two on anterior cingulate cortex activity elicited by a Go/NoGo response inhibition task in healthy volunteers [Passamonti et al., 2008[. The lack of interaction between 5HTTLPR and MAOA in our study may in part reflect the fact that while MAOA is more abundant in catecholaminergic neurons, MAOB is more abundant in serotonergic neurons [Levitt et al., 1982; Saura et al., 1992[.
Although the lack of cognitive effects of 5HTTLPR were predictable given the cognitive domains assessed, it is somewhat surprising that no main effect was detected for MAOA. The negative main effects of MAOA reported here are in contrast to previous reports of IQ effects in a sample of healthy Chinese females [Yu et al., 2005[, two samples of autistic children from Israel [Yirmiya et al., 2002[ and Canada [Cohen et al., 2003[, and a sample of boys with ADHD from Israel, where the MAOA low activity allele was associated with better performance on a continuous performance test [Manor et al., 2002[. There are a number of differences between this and the previous, positive reports including ethnicity, sex distribution, and developmental stage, which might conceivably affect the genetic effect on cognition among samples. For example, MAOA's effects may be limited to one particular developmental stage, or in previous reports may have been due to a second locus that shows differential linkage disequilibrium between ethnic groups. The sex-specific analyses presented here reduced statistical power through halving the sample size (reducing to around 69% the power to detect an association explaining 0.2% of cognitive variance). Nonetheless, this sample remains the largest so far examined with respect to MAOA and cognition, and the complete lack of difference in cognitive score between genotypes in either sex is again a decisive negative result.
In contrast to the absence of main effects of MAOA, there was a modest but significant interactive effect of MAOA genotype and COMT genotype on working memory performance in boys but not girls. With few exceptions, cognitive genetic studies have thus far had inadequately sized samples to realistically assess possible gene–gene (epistatic) interactions. The COMT × MAOA interaction observed here for working memory is plausible given the dependence of working memory on prefrontal dopaminergic availability. That no similar effect was found for IQ is surprising: COMT genotype has a strong, male-specific effect on verbal IQ in this sample [Barnett et al., 2007[, and previous evidence has supported an effect of MAOA on IQ but not measures of executive function [Yu et al., 2005[.
Separate analyses were carried out for boys and girls and for multiple cognitive phenotypes. Since scores on the four cognitive measures were correlated, strict correction for multiple comparisons using, for example, a Bonferroni correction, was not suitable. Nonetheless, while the large sample used in the study reduces the chances of Type II error, the possibility of Type I error remains present.
While both tasks that have a high working memory content [Owen, 1997; Owen et al., 2005[ and those that load highly on general intelligence [Duncan et al., 2000[ typically activate a range of lateral frontal cortical areas, there are nonetheless task-specific differences in cognitive demands. Plausible explanations for the association between catecholaminergic genes and working memory, but not IQ, therefore involve the specific neural underpinnings of the different tasks, and the complexity of gene expression within different regions of the brain. While MAOA has a widespread distribution in the brain [Grimsby et al., 1990[, it has recently been suggested that frontal cortical MAOA expression may be predominantly determined by genetic and epigenetic factors other than this VNTR, including a currently unknown locus with which it shows strong linkage disequilibrium [Balciuniene et al., 2002; Pinsonneault et al., 2006[. Similarly, while COMT expression appears greater in PFC than other regions [Matsumoto et al., 2003[, recent studies have shown that the expression of gene products of COMT is complex, with tissue-specific monoallelic expression [Gimelbrant et al., 2007[, multiple mRNA variants expressed at different levels across the brain [Tunbridge et al., 2007a[, and differences in both mRNA and protein expression across developmental stages [Tunbridge et al., 2007a,b[. It is also important to note that variation at any one locus in a gene may not act alone in exerting cognitive effects. For example, we have recently reported the effects of additional COMT SNPs that act in concert with the Val158Met polymorphism in influencing cognitive function in this sample [Barnett et al., 2009[. While the inclusion of additional SNPs further reduces group sizes, and hence statistical power, the more accurate functional characterization gained by haplotype analysis may sometimes offset this loss of power. In this study, re-analysis using COMT haplotype groups produced no meaningful change in results from those seen using just the Val158Met genotype (see Supplementary Fig. 1 for the effects of COMT haplotype characterization of the COMT × MAOA interaction on boys' working memory).
The gene–gene interaction found here suggests that MAOA genotype affects working memory only in the context of high prefrontal catecholamine availability. There is, of course, a distinction between demonstrating a statistical interaction, and understanding the biological mechanism of interaction between two genes [Clayton and McKeigue, 2001[. Further studies will clearly be needed both to replicate this effect on working memory and to clarify the biological pathway of any such interaction. While prefrontal dopaminergic function is one obvious candidate, there may be other means by which COMT and/or MAOA expression affect cognitive function. One such possibility is NA, which, like DA, is catabolized to some extent by both COMT and MAOA. At present, dopaminergic pathways remain the more obvious candidate, because COMT appears to play a relatively minor role in regulating NA in the PFC [Gogos et al., 1998; Tunbridge et al., 2004[.
The sex-specific nature of COMT × MAOA effect on working memory was in accordance with our previous study [Barnett et al., 2007[, which showed that COMT genotype affected a wide range of cognitive functions in boys but had no effect in girls. There is additional wide-ranging evidence to suggest that the effects of COMT may be different between the sexes [Harrison and Tunbridge, 2008[, for example, DA levels in the frontal cortex are affected only in male COMT knockout mice, which show sex-specific behavioral phenotypes [Gogos et al., 1998[, and there are sex differences in both the expression and effects of COMT in human PFC [Chen et al., 2004[. Studies in model organisms, where our understanding of genetic variation is further advanced than in humans, confirm the existence of a wide range of sexually dimorphic genetic effects on normal variation in traits such as lifespan, obesity, and skeletal form [Nuzhdin et al., 1997; Farber and Medrano, 2007[. The same appears true in humans where there are known sex-specific effects on reproductive, physiological, and disease traits, probably resulting from differential gene regulation in males and females [Ober et al., 2008[. The likely explanation for sex-specific associations with COMT are the bilateral relationships between COMT and estrogen-related compounds: estrogens mediate COMT expression [Xie et al., 1999[, and COMT metabolizes catechol estrogens, a process regulated by Val158Met variation [Worda et al., 2003[. In addition to these issues with COMT, the location of the MAOA gene on the X chromosome complicates association studies, with the result that little is known about the relative expression and effects of MAOA in the two sexes.
In conclusion, we present evidence that functional polymorphisms in COMT and MAOA, two genes involved in the functional deactivation of DA and other neurotransmitters, show interactive effects on working memory performance in normal children from a large, homogeneous, and representative sample. While neither gene has been unquestionably linked to psychiatric disorder, normal variation in brain function is likely to be partly determined by genetic variation in neurotransmitter pathways. Understanding genetic effects on normal brain function is a necessary stage in understanding the basis of abnormal function, but large samples are required to tease out relatively subtle effects on cognitive development at the population level.
Acknowledgments
The authors thank all of the families who participated in the study, the midwives for their help in recruiting the families, and the entire Avon Longitudinal Study of Parents and Children team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. Core support for the Avon Longitudinal Study of Parents and Children is provided by the UK Medical Research Council, the Wellcome Trust, and the University of Bristol. Dr. Barnett was funded by the Stanley Medical Research Institute and the Parke Davis Exchange Fellowship. Dr. Jones receives funding from the Wellcome Trust (grant number 074296/Z/04/Z); this work forms part of the NIHR CLAHRC for Cambridgeshire & Peterborough.
Conflict of interest
Dr. Barnett is an employee of Cambridge Cognition Ltd and a co-inventor on patent PCT/GB2005/003279 (methods for assessing psychotic disorders) with Dr. Jones. Dr. Jones has also received research grant support from GlaxoSmithKline and has received a speaker's honorarium from Eli Lilly. Dr. Heron, Dr. Goldman, and Dr. Xu report no competing interests.
REFERENCES
- Araya R, Hu X, Heron J, Enoch MA, Evans J, Lewis G, et al. Effects of stressful life events, maternal depression and 5-HTTLPR genotype on emotional symptoms in pre-adolescent children. Am J Med Genet Part B. 2009;150B:670–682. doi: 10.1002/ajmg.b.30888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Balciuniene J, Emilsson L, Oreland L, Pettersson U, Jazin E. Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet. 2002;110:1–7. doi: 10.1007/s00439-001-0652-8. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Heron J, Goldman D, Jones PB, Xu K. Effects of catechol-O-methyltransferase on normal variation in the cognitive function of children. Am J Psychiatry. 2009;166:909–916. doi: 10.1176/appi.ajp.2009.08081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Heron J, Ring SM, Golding J, Goldman D, Xu K, Jones PB. Gender-specific effects of the catechol-O-methyltransferase Val108/158Met polymorphism on cognitive function in children. Am J Psychiatry. 2007;164:142–149. doi: 10.1176/ajp.2007.164.1.142. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. J Abnorm Psychol. 2007;116:208–212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Borg J, Henningsson S, Saijo T, Inoue M, Bah J, Westberg L, et al. Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. Int J Neuropsychopharmacol. 2009;12:783–792. doi: 10.1017/S1461145708009759. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Carlson S, Tanila H, Rama P, Mecke E, Pertovaara A. Effects of medetomidine, an alpha-2 adrenoceptor agonist, and atipamezole, an alpha-2 antagonist, on spatial memory performance in adult and aged rats. Behav Neural Biol. 1992;58:113–119. doi: 10.1016/0163-1047(92)90327-z. [DOI] [PubMed] [Google Scholar]
- Case R, Kurland DM, Goldberg J. Operational efficiency and the growth of short-term-memory span. J Exp Child Psychol. 1982;33:386–404. [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Roiser JP, Cools R, Rubinsztein DC, Sahakian BJ, Robbins TW. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: Implications for the 5-HT theory of impulsivity. Psychopharmacology (Berl) 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clayton D, McKeigue PM. Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet. 2001;358:1356–1360. doi: 10.1016/S0140-6736(01)06418-2. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Liu X, Schutz C, White BN, Jenkins EC, Brown WT, Holden JJ. Association of autism severity with a monoamine oxidase A functional polymorphism. Clin Genet. 2003;64:190–197. doi: 10.1034/j.1399-0004.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- Crisan LG, Pana S, Vulturar R, Heilman RM, Szekely R, Druga B, et al. Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Soc Cogn Affect Neurosci. 2009;4(4):399–408. doi: 10.1093/scan/nsp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha FF, Malloy-Diniz L, Lage NV, Romano-Silva MA, de Marco LA, Correa H. Decision-making impairment is related to serotonin transporter promoter polymorphism in a sample of patients with obsessive-compulsive disorder. Behav Brain Res. 2008;195:159–163. doi: 10.1016/j.bbr.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Devlin B, Daniels M, Roeder K. The heritability of IQ. Nature. 1997;388:468–471. doi: 10.1038/41319. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, et al. A neural basis for general intelligence. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallgatter AJ, Jatzke S, Bartsch AJ, Hamelbeck B, Lesch KP. Serotonin transporter promoter polymorphism influences topography of inhibitory motor control. Int J Neuropsychopharmcol. 1999;2:115–120. doi: 10.1017/S1461145799001455. [DOI] [PubMed] [Google Scholar]
- Farber CR, Medrano JF. Fine mapping reveals sex bias in quantitative trait loci affecting growth, skeletal size and obesity-related traits on mouse chromosomes 2 and 11. Genetics. 2007;175:349–360. doi: 10.1534/genetics.106.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RB, Van Kammen DP, Peters JL, Rosen J, Van Kammen WB, Nugent A, et al. Clonidine improves memory function in schizophrenia independently from change in psychosis. Preliminary findings. Schizophr Res. 1988;1:417–423. doi: 10.1016/0920-9964(88)90024-2. [DOI] [PubMed] [Google Scholar]
- Firk C, Markus CR. Differential effects of 5-HTTLPR genotypes on mood, memory, and attention bias following acute tryptophan depletion and stress exposure. Psychopharmacology (Berl) 2009;203:805–818. doi: 10.1007/s00213-008-1428-9. [DOI] [PubMed] [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: Biased attention and the human serotonin transporter gene. Proc Biol Sci. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimelbrant A, Hutchinson JN, Thompson BR, Chess A. Widespread monoallelic expression on human autosomes. Science. 2007;318:1136–1140. doi: 10.1126/science.1148910. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Pembrey M, Jones R. ALSPAC—The Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Green AR, Youdim MB. Effects of monoamine oxidase inhibition by clorgyline, deprenil or tranylcypromine on 5-hydroxytryptamine concentrations in rat brain and hyperactivity following subsequent tryptophan administration. Br J Pharmacol. 1975;55:415–422. doi: 10.1111/j.1476-5381.1975.tb06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby J, Lan NC, Neve R, Chen K, Shih JC. Tissue distribution of human monoamine oxidase A and B mRNA. J Neurochem. 1990;55:1166–1169. doi: 10.1111/j.1471-4159.1990.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-Methyltransferase (COMT): A gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33(13):3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Homberg JR, van den Bos R, den Heijer E, Suer R, Cuppen E. Serotonin transporter dosage modulates long-term decision-making in rat and human. Neuropharmacology. 2008;55:80–84. doi: 10.1016/j.neuropharm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Jones RW, Ring S, Tyfield L, Hamvas R, Simmons H, Pembrey M, et al. A new human genetic resource: A DNA bank established as part of the Avon longitudinal study of pregnancy and childhood (ALSPAC) Eur J Hum Genet. 2000;8:653–660. doi: 10.1038/sj.ejhg.5200502. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: Reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kraft JB, Slager SL, McGrath PJ, Hamilton SP. Sequence analysis of the serotonin transporter and associations with antidepressant response. Biol Psychiatry. 2005;58:374–381. doi: 10.1016/j.biopsych.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA. 1982;79:6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: Regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Mair RG, McEntee WJ. Cognitive enhancement in Korsakoff's psychosis by clonidine: A comparison with L-dopa and ephedrine. Psychopharmacology (Berl) 1986;88:374–380. doi: 10.1007/BF00180841. [DOI] [PubMed] [Google Scholar]
- Manly T, Anderson V, Nimmo-Smith I, Turner A, Watson P, Robertson IH. The differential assessment of children's attention: The Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. J Child Psychol Psychiatry. 2001;42:1065–1081. doi: 10.1111/1469-7610.00806. [DOI] [PubMed] [Google Scholar]
- Manor I, Tyano S, Mel E, Eisenberg J, Bachner-Melman R, Kotler M, Ebstein RP. Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): Preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA) Mol Psychiatry. 2002;7:626–632. doi: 10.1038/sj.mp.4001037. [DOI] [PubMed] [Google Scholar]
- Mantere T, Tupala E, Hall H, Sarkioja T, Rasanen P, Bergstrom K, et al. Serotonin transporter distribution and density in the cerebral cortex of alcoholic and nonalcoholic comparison subjects: A whole-hemisphere autoradiography study. Am J Psychiatry. 2002;159:599–606. doi: 10.1176/appi.ajp.159.4.599. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, et al. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003;28:1521–1530. doi: 10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: A meta-analysis. Mol Psychiatry. 2010;15(9):918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J Comp Neurol. 2003;466:478–494. doi: 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- Nuzhdin SV, Pasyukova EG, Dilda CL, Zeng ZB, Mackay TF. Sex-specific quantitative trait loci affecting longevity in Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94:9734–9739. doi: 10.1073/pnas.94.18.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. 1994;33:575–588. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Gioia MC, Magariello A, Muglia M, Quattrone A, Fera F. Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage. 2008;40:1264–1273. doi: 10.1016/j.neuroimage.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Payton A, Gibbons L, Davidson Y, Ollier W, Rabbitt P, Worthington J, et al. Influence of serotonin transporter gene polymorphisms on cognitive decline and cognitive abilities in a nondemented elderly population. Mol Psychiatry. 2005;10:1133–1139. doi: 10.1038/sj.mp.4001733. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Papp AC, Sadee W. Allelic mRNA expression of X-linked monoamine oxidase a (MAOA) in human brain: Dissection of epigenetic and genetic factors. Hum Mol Genet. 2006;15:2636–2649. doi: 10.1093/hmg/ddl192. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, de Martino B, Tan GC, Kumaran D, Seymour B, Wood NW, Dolan RJ. A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci. 2009;29:5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Muller U, Clark L, Sahakian BJ. The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. Int J Neuropsychopharmacol. 2007;10(4):449–461. doi: 10.1017/S146114570600705X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro41-1049 and 3H-Ro19-6327 in vitro: Localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12:1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A, Dreisbach G, Muller J, Goschke T, Brocke B, Lesch KP. Genetic variation of serotonin function and cognitive control. J Cogn Neurosci. 2007;19:1923–1931. doi: 10.1162/jocn.2007.19.12.1923. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Watson DR, Barrett SL, Cooper SJ. Rapid tryptophan depletion improves decision-making cognition in healthy humans without affecting reversal learning or set shifting. Neuropsychopharmacology. 2006;31:1519–1525. doi: 10.1038/sj.npp.1300980. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Lane TA, Harrison PJ. Expression of multiple catechol-o-methyltransferase (COMT) mRNA variants in human brain. Am J Med Genet Part B. 2007a;144B(6):834–839. doi: 10.1002/ajmg.b.30539. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2007b;17:1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Golombok S, Rust J. Wechsler Intelligence Scale for Children—Third Edition UK Manual. Sidcup, UK: The Psychological Corporation; 1992. [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Rose RM, Abell CW. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience. 1988;25:439–456. doi: 10.1016/0306-4522(88)90250-3. [DOI] [PubMed] [Google Scholar]
- Willoughby J, Glover V, Sandler M. Histochemical localisation of monoamine oxidase A and B in rat brain. J Neural Transm. 1988;74:29–42. doi: 10.1007/BF01243573. [DOI] [PubMed] [Google Scholar]
- Worda C, Sator MO, Schneeberger C, Jantschev T, Ferlitsch K, Huber JC. Influence of the catechol-O-methyltransferase (COMT) codon 158 polymorphism on estrogen levels in women. Hum Reprod. 2003;18:262–266. doi: 10.1093/humrep/deg059. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Pilowsky T, Tidhar S, Nemanov L, Altmark L, Ebstein RP. Family-based and population study of a functional promoter-region monoamine oxidase A polymorphism in autism: Possible association with IQ. Am J Med Genet. 2002;114:284–287. doi: 10.1002/ajmg.10189. [DOI] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Hong CJ, Chen MC, Yang CW, Chen TJ. Association study of a functional MAOA-uVNTR gene polymorphism and cognitive function in healthy females. Neuropsychobiology. 2005;52:77–82. doi: 10.1159/000086609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The number of children included in each analysis varied by the availability of cognitive data and genotypes: cognitive data were available on around two-thirds of those for whom DNA is available. As previously reported [Araya et al., 2009[, after stringent quality control measures, fewer children were available for inclusion in analyses involving 5HTTLPR genotype. There were no cognitive differences between children for whom 5HTTLPR, MAOA, or COMT genotypes were or were not available (all P > 0.05). Allele frequencies were, for 5HTTLPR alleles S = 41.4%, LA = 51.5%, LG = 7.1%, and for MAOA, 2-repeat = 0.2%, 3-repeat = 34.0%, 3.5-repeat = 2.0%, 4-repeat = 62.4%, 5-repeat = 1.5%. COMT allele frequencies, as previously reported, were Val = 48.6%, Met = 51.4%. The number of children considered as low, medium, and high activity genotypes for whom at least one cognitive measure was available are shown in Table II.
TABLE II.
Sample Sizes of High, Medium, and Low Activity MAOA, 5HTTLPR, and COMT Genotype Groups in ALSPAC Cognitive Study
| High activity | Medium activity | Low activity | ||||
|---|---|---|---|---|---|---|
| Total (n) | Genotypes | n | Genotypes | n | Genotypes | n |
| MAOA girls | ||||||
| 2,984 | 3.5/3.5; 3.5/4; 4/4 | 1,245 | 3/3.5; 3/4 | 1,380 | 3/3 | 359 |
| MAOA boys | ||||||
| 3,066 | 3.5; 4 | 2,037 | NA | 0 | 3 | 1,029 |
| 5HTTLPR | ||||||
| 4,579 | LALA | 1,153 | SLA; LGLA | 2,346 | SS; SLG; LGLG | 1,080 |
| COMT | ||||||
| 5,909 | Val/Val | 1,396 | Val/Met | 2,921 | Met/Met | 1,592 |
In each case “high activity” refers to the allele resulting in faster clearance of synaptic neurotransmitter.


