Abstract
We retrospectively analyzed clinical outcomes of patients aged 18–59 with cytogenetically normal AML (CN-AML) and mutations in the FLT3 receptor according to type of postremission therapy. Specifically, we compared the outcomes of patients who underwent autologous SCT versus consolidation chemotherapy. There were 37 patients with an ITD mutation (7 also had a TKD mutation) and 19 patients with an isolated TKD mutation at diagnosis. In all, patients with an isolated TKD (n=16) had an improved DFS and OS (p=.031 and .014, respectively) compared to ITD patients (n=21). For individuals with an isolated TKD mutation, survival outcomes were similar irrespective of the type of postremission therapy (n=7 for SCT and 9 for chemotherapy) (p=0.97 and 0.082, respectively). However, ITD positive patients who underwent an SCT in CR1 (n=10) had an improved DFS but similar OS compared to those who received consolidation chemotherapy (n=11) (p=.05 and .27). These results suggest that high dose chemotherapy with autologous SCT may be a reasonable therapeutic choice over consolidation chemotherapy for young CN-AML patients with a FLT3ITD mutation.
Acute myeloid leukemia (AML) is a relatively resistant myeloid neoplasm commonly occurring in older patients (median age at diagnosis is 67 years).(1) AML outcomes are heterogeneous, with cure rates ranging from essentially zero in subsets of older patients to 80% in younger patients with favorable disease features.(2) Among various risk factors, karyotype is an important independent risk factor and is used to divide AML into good, intermediate and poor prognostic groups.(3, 4) The normal karyotype group is considered to be at intermediate risk and constitutes approximately 40% of patients with AML. However, even these patients have a heterogeneous outcome, with OS rates ranging from 24% to 42%. This group may be further stratified according to the presence or absence of mutations or expression levels of various molecular markers. (5, 6)
FLT3ITD represents a segmental duplication within the juxtamembrane domain of FLT3 receptor and is found in 30–40% of CN-AML patients.(8) FLT3ITD is associated with monocytoid differentiation (FAB M5), higher white blood cell counts, increased serum lactate dehydrogenase, and higher peripheral and bone marrow blasts compared to wild-type FLT3 (wt FLT3) CN-AML patients.(9, 10) Patients with this mutation have similar complete remission (CR) rates but significantly increased relapse rates and a reduced OS as compared to patients with wt FLT3.(9–11) It has been further identified that the allelic ratio of mutant:wt transcripts impacts prognosis.(8, 10, 12–14) The length of the duplicated fragment and its specific location within FLT3 may also affect prognosis.(13, 15–17) Overall, CN-AML patients with a FLT3ITD mutation have an inferior prognosis compared to those with a wtFLT3. There is considerable debate over the best postremission treatment for FLT3ITD CN-AML patients.(18, 19)
Another mutation, FLT3TKD, is a point mutation in the tyrosine kinase domain of the receptor and is found in approximately 10% of all CN-AML patients.(8) Similar to patients with FLT3ITD mutations, patients with FLT3TKD also have an elevated white blood cell count at presentation. However, the prognostic significance of TKD mutations is unclear.(9–11, 22–24)
We analyzed patients with CN-AML and mutated FLT3 to determine the relationship between different postremission therapies and outcome.
Our study includes all consecutively treated patients at our institutions who consented for determination of their FLT3 mutation status at diagnosis on a research study. The choice of post-remission therapy was not randomized but instead reflects the availability of open protocols and physician and patient preference.
There were a total of 37 patients who were below the age of 60 years at diagnosis and had a normal karyotype with a FLT3ITD. Treatment histories of these patients are described in supplemental material (Fig S1). Patients who were positive for both the ITD and TKD mutation were included in the ITD group (n=7) because these individuals have similar outcomes as those with an isolated ITD mutation.(23, 27) The remission frequency for this patient population was 81.1% (30/37). There were 19 newly diagnosed AML patients with an isolated TKD mutation who were under the age of 60 and had a normal karyotype. Their treatment histories are shown in Supplemental Figure 2 (Fig S2). The CR frequency for this group was 89.5% (17/19).
Excluded from our research question were 4/37 patients with FLT3ITD and 1/19 with FLT3TKD who received allogeneic SCT in CR1. However, we included patients who received an allogeneic SCT after first relapse (ITD: n=5; TKD: n=3). Time dependent covariate analysis indicated that receiving an allogeneic SCT after relapse did not significantly improve OS (p=0.62 for the ITD group and 0.83 for the entire cohort of patients; there were too few patients in the TKD group for this analysis to be conducted).
Patient characteristics such as age at diagnosis, time to CR1, WBC at diagnosis and gender are in presented in Table 1. These features were balanced between those who were assigned to autologous SCT or consolidation chemotherapy in both ITD and TKD patient groups. Also, there was no significant difference in the distribution of allelic ratio and ITD length within the two treatment groups for ITD patients (p=.22 and .2, respectively). We then studied the relationship between baseline characteristics and clinical outcome and we found that among the characteristics listed in Table 1, only female gender was associated with a better DFS in patients with an isolated TKD mutation (p=.03).
Table 1.
Patient characteristics for ITD and TKD positive patients who initiated autologous SCT or consolidation chemotherapy. P value corresponds to comparisons within each type of mutation and not across FLT3 mutation types.
| ITD positive patients | TKD positive patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Autologous SCT | Chemotherapy | P value | Autologous SCT | Chemotherapy | P value | |||||
| N | Median, range | N | Median, range |
N | Median, range |
N | Median, range |
|||
| Age at dx | 10 | 48 (29, 59) | 11 | 47 (21, 60) | 0.97 | 7 | 49 (39, 60) | 9 | 46 (23, 59) | 0.22 |
| Duration from diagnosis to CR1 (months) | 10 | 1.35 (0.99, 2.07) | 11 | 1.18 (0.95, 2.63) | 7 | 1.2 (0.9, 1.8) | 9 | 1.2 (0.9, 1.9) | ||
| WBC | 9 | 58.7 (0.9, 150.2) | 11 | 52.9 (13.1, 149.9) | 0.96 | 7 | 27 (16, 238.6) | 9 | 10.1 (2.0, 53.9) | 0.16 |
| Gender | 0.65 | 0.1 | ||||||||
| Female | 5 | 7 | 3 | 8 | ||||||
| Male | 5 | 3 | 4 | 1 | ||||||
| ITD length | 10 | 40.5 (18, 210) | 11 | 27 (17–165) | 0.2 | |||||
| Allelic Ratio | 10 | .27 (.02,1.17) | 11 | .32 (.16–1.32) | 0.22 | |||||
We also found that an allelic ratio of >.2 was associated with a worse DFS and OS in ITD mutation positive patients who had initiated postremission autologous SCT or chemotherapy (n=21) (p=0.01 and 0.04). A value of 0.2 was determined as the optimal cut point by recursive partitioning analysis. The median length of the ITD in patients who initiated postremission autologous SCT or chemotherapy was 33bps (n=21) (Range=17–210). For patients with more than one mutation (n=5), the length of the most dominant mutation was chosen for analysis. In our study, the ITD length was not associated with DFS or OS. No other variable was noted to influence DFS or OS.
We next analyzed the survival of patients with an isolated TKD mutation according to type of postremission therapy. For the 7 patients who initiated autologous SCT compared to the 9 patients who initiated consolidation chemotherapy, there was no significant difference in DFS or OS (p=0.97 and 0.082, respectively). Similarly, there was no significant difference in DFS or OS for patients who received a stem cell infusion or at least 3 cycles of consolidation chemotherapy (n=6 and 8, respectively; p=0.78 and 0.25, respectively).
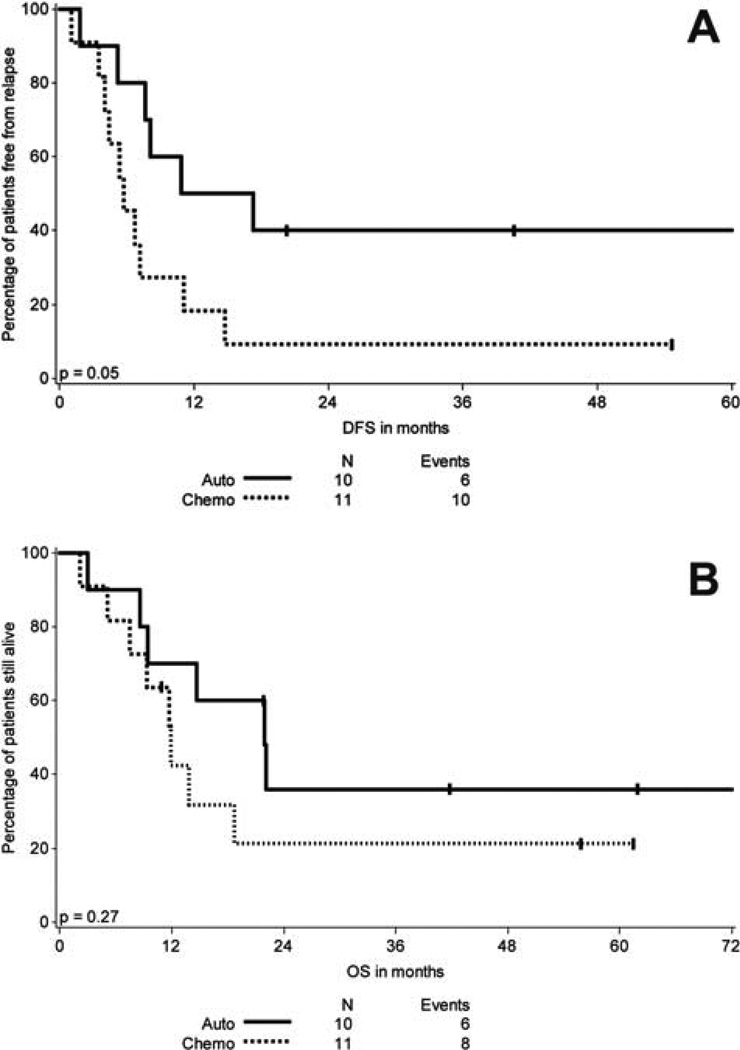
On the other hand, patients with an ITD mutation who initiated autologous SCT (n=10) had an improved DFS compared to those who initiated consolidation chemotherapy (n=11) (p=0.05) (Figure IA), however there was no difference in OS (p=.27) (Figure IB). Since some patients in each cohort (n=2 for autologous SCT and n=2 for consolidation chemotherapy) did not complete the initiated postremission therapy, defined as having received infusion of stem cells or at least 3 cycles of chemotherapy, respectively, we then compared the survival in patients who had completed their postremission therapy as defined above. Again, we found that autologous SCT (n=8) was significantly associated with an improved DFS compared to consolidation chemotherapy (n=9) (p=.03) while no significant difference in OS was observed (p=.19).
Figure 1.
A. DFS in months of patients with FLT3R ITD according to type of postremission therapy initiated
B. OS in months of patients with FLT3R ITD according to type of postremission therapy initiated
Lastly we compared the survival outcomes of patients with ITD versus TKD who achieved CR and progressed to postremission therapy, excluding those who underwent allogeneic SCT in CR1. Patients with an isolated TKD mutation (n=16) had a significantly longer DFS and OS compared to patients with ITD (n=21) (p=.031 and .014).
In summary, we analyzed the outcome of AML patients under 60 years with normal cytogenetics and FLT3 mutations according to the type of postremission therapy they initiated or received. Younger adults with ITD who initiated or received autologous SCT had an improved DFS compared to consolidation chemotherapy. OS, however, was not different for the two treatment modalities. For patients with isolated TKD, there was no difference in DFS or OS of patients who received autologous SCT versus consolidation chemotherapy.
There is considerable debate about the optimal postremission therapy for CN-AML patients with a FLT3ITD mutation. Current options vary among allogeneic SCT, autologous SCT, and/or chemotherapy along with use of novel FLT3R inhibitors. In general, comparisons of autologous SCT and chemotherapy have not shown a clear difference in outcome when all AML patients are included. The potential benefit of autologous SCT in AML subgroups has not been extensively studied. Yoshimoto et al., in a retrospective analysis, demonstrated a similar DFS and OS for patients with a wtFLT3 versus a FLT3ITD who underwent an autologous SCT in CR1.(28) Bornhauser et al. also noted a decrease in probability of relapse and an improvement in OS for patients with FLT3ITD who received an autologous SCT in CR1 compared to those receiving chemotherapy alone.(19) In contrast, Schlenk et al. found no difference in relapse free survival and OS between normal cytogenetic patients receiving autologous SCT or conventional consolidation chemotherapy in both FLT3ITD and wt FLT3 patient groups.(18)
Our data raise the question as to whether autologous SCT may be considered over consolidation chemotherapy for patients with ITD who are not undergoing allogeneic SCT or therapy with a FLT3 inhibitor.
MATERIALS and METHODS
Patients
We conducted a retrospective analysis of all patients who presented to the Dana Farber Cancer Institute/Brigham and Women Hospital and Massachusetts General Hospital between 2002 and 2008 who underwent testing for FLT3 mutations. It was the policy to test all patients with newly diagnosed AML in this fashion. Patients had consented to an IRB approved research protocol prior to obtaining samples for FLT3 analysis.
We analyzed outcomes for all adult patients with FLT3 mutations who were newly diagnosed, had a normal karyotype and were < 60 years of age. CR rates, disease free survival, overall survival and type of treatment received were assessed by medical record review.
Mutations Analysis
FLT3 mutations, ITD length, sequence and allelic ratio, were determined as previously described.(25) Nucleophosmin gene (NPM1) mutation status is not available.
End Points and Definitions
CR was defined by the presence of a normocellular bone marrow containing < 5 percent blasts and showing trilineage maturation with an absolute neutrophil count of more than 1000/ul and a platelet count of more than 100,000/ul.(26) DFS was defined as the time from the date of first CR to an event (death in first CR or relapse). OS was defined as the time from diagnosis to date of death. Patients lost to follow-up were censored at the date of last documented contact.
Statistics
The two sample t-test and the Fisher exact test were used to assess the significance of differences in clinical parameters between the patient groups. The method of Kaplan and Meier was used to estimate DFS and OS; groups were compared using the log-rank test. The Cox proportional hazards regression model was used to explore associations between therapeutic and demographic characteristics and DFS or OS in a multivariable setting. Receipt of allogeneic transplant in the setting of relapse or CR2 or higher was analyzed as a time-varying covariate in these models. A p-value less than 0.05 was interpreted as statistically significant. There was no adjustment for multiple comparisons.
Supplementary Material
Supplementary Information
Fig S1 Distribution of patients with FLT3RITD (+/− TKD) mutations according to treatment
Fig S2 Distribution of patients with isolated FLT3R TKD mutations according to treatment
Acknowledgments
Harshabad Singh is supported by the Judith Keen Postdoctoral Research Fellowship. This research is funded in part by the Leukemia Discovery and Treatment Fund at Massachusetts General Hospital.
Funding: Harshabad Singh is supported by the Judith Keen Postdoctoral Research Fellowship. This research is funded in part by the Leukemia Discovery and Treatment Fund at Massachusetts General Hospital.
Footnotes
Conflict of Interest
Dr. Richard M Stone has served as a consultant for Novartis
REFERENCES
- 1.Zander AR, Bacher U, Finke J. Allogeneic stem cell transplantation in acute myeloid leukemia: establishment of indications on the basis of individual risk stratification. Dtsch Arztebl Int. 2008;105:663–669. doi: 10.3238/arztebl.2008.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 3.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 4.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, Koduru PR, Moore JO, Stone RM, Mayer RJ, Feldman EJ, Davey FR, Schiffer CA, Larson RA, Bloomfield CD. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 5.Gregory TK, Wald D, Chen Y, Vermaat JM, Xiong Y, Tse W. Molecular prognostic markers for adult acute myeloid leukemia with normal cytogenetics. J Hematol Oncol. 2009;2:23. doi: 10.1186/1756-8722-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attar EC, Maharry K, Mrozek K, Radmacher MD, Whitman SP, Paschka P, Langer C, Holland KB, Powell BL, Kolitz JE, Carroll AJ, Larson RA, Marcucci G, Bloomfield CD. Increased Expression of Macrophage Migration Inhibitory Factor (MIF) Receptor CD74 Is Associated with Inferior Outcome in Younger Patients (Pts) with Cytogenetically Normal Acute Myeloid Leukemia (CN-AML): a Cancer and Leukemia Group B (CALGB) Study. ASH Annual Meeting Abstracts. 2009;114:1616. -. [Google Scholar]
- 7.Zheng R, Small D. Mutant FLT3 signaling contributes to a block in myeloid differentiation. Leuk Lymphoma. 2005;46:1679–1687. doi: 10.1080/10428190500261740. [DOI] [PubMed] [Google Scholar]
- 8.Meshinchi S, Appelbaum FR. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009;15:4263–4269. doi: 10.1158/1078-0432.CCR-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, Dohner H, Dohner K. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 10.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, Wermke M, Bornhauser M, Ritter M, Neubauer A, Ehninger G, Illmer T. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 11.Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia. 2005;19:1345–1349. doi: 10.1038/sj.leu.2403838. [DOI] [PubMed] [Google Scholar]
- 12.Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, Kaspers GJ, Heerema NA, Gerbing R, Lange BJ, Radich JP. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, Linch DC. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 14.Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, Carroll AJ, Mrozek K, Vardiman JW, George SL, Kolitz JE, Larson RA, Bloomfield CD, Caligiuri MA. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 15.Kusec R, Jaksic O, Ostojic S, Kardum-Skelin I, Vrhovac R, Jaksic B. More on prognostic significance of FLT3/ITD size in acute myeloid leukemia (AML) Blood. 2006;108:405–406. doi: 10.1182/blood-2005-12-5128. author reply 406. [DOI] [PubMed] [Google Scholar]
- 16.Stirewalt DL, Kopecky KJ, Meshinchi S, Engel JH, Pogosova-Agadjanyan EL, Linsley J, Slovak ML, Willman CL, Radich JP. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107:3724–3726. doi: 10.1182/blood-2005-08-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayser S, Schlenk RF, Londono MC, Breitenbuecher F, Wittke K, Du J, Groner S, Spath D, Krauter J, Ganser A, Dohner H, Fischer T, Dohner K. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386–2392. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]
- 18.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, Habdank M, Spath D, Morgan M, Benner A, Schlegelberger B, Heil G, Ganser A, Dohner H. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 19.Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109:2264–2265. doi: 10.1182/blood-2006-09-047225. author reply 2265. [DOI] [PubMed] [Google Scholar]
- 20.Doubek M, Muzik J, Szotkowski T, Koza V, Cetkovsky P, Kozak T, Zak P, Voglova J, Struncova S, Dusek L, Indrak K. Is FLT3 internal tandem duplication significant indicator for allogeneic transplantation in acute myeloid leukemia? An analysis of patients from the Czech Acute Leukemia Clinical Register (ALERT) Neoplasma. 2007;54:89–94. [PubMed] [Google Scholar]
- 21.Gale RE, Hills R, Kottaridis PD, Srirangan S, Wheatley K, Burnett AK, Linch DC. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106:3658–3665. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 22.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters--an analysis of 3082 patients. Blood. 2008;111:2527–2537. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 23.Moreno I, Martin G, Bolufer P, Barragan E, Rueda E, Roman J, Fernandez P, Leon P, Mena A, Cervera J, Torres A, Sanz MA. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88:19. [PubMed] [Google Scholar]
- 24.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, Asou N, Kuriyama K, Yagasaki F, Shimazaki C, Akiyama H, Saito K, Nishimura M, Motoji T, Shinagawa K, Takeshita A, Saito H, Ueda R, Ohno R, Naoe T. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong SA, Mabon ME, Silverman LB, Li A, Gribben JG, Fox EA, Sallan SE, Korsmeyer SJ. FLT3 mutations in childhood acute lymphoblastic leukemia. Blood. 2004;103:3544–3546. doi: 10.1182/blood-2003-07-2441. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Bagrintseva K, Geisenhof S, Kern R, Eichenlaub S, Reindl C, Ellwart JW, Hiddemann W, Spiekermann K. FLT3-ITD-TKD dual mutants associated with AML confer resistance to FLT3 PTK inhibitors and cytotoxic agents by overexpression of Bcl-x(L) Blood. 2005;105:3679–3685. doi: 10.1182/blood-2004-06-2459. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimoto G, Nagafuji K, Miyamoto T, Kinukawa N, Takase K, Eto T, Kato K, Hayashi S, Kamimura T, Ohno Y, Taniguchi S, Harada M. FLT3 mutations in normal karyotype acute myeloid leukemia in first complete remission treated with autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2005;36:977–983. doi: 10.1038/sj.bmt.1705169. [DOI] [PubMed] [Google Scholar]
- 29.Stone RM, Fischer T, Paquette R, Schiller G, Schiffer CA, Ehninger G, Cortes J, Kantarjian HM, DeAngelo DJ, Huntsman-Labed A, Dutreix C, Rai S, Giles F. A Phase 1b Study of Midostaurin (PKC412) in Combination with Daunorubicin and Cytarabine Induction and High-Dose Cytarabine Consolidation in Patients Under Age 61 with Newly Diagnosed De Novo Acute Myeloid Leukemia: Overall Survival of Patients Whose Blasts Have FLT3 Mutations Is Similar to Those with Wild-Type FLT3. ASH Annual Meeting Abstracts. 2009;114:634. -. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Fig S1 Distribution of patients with FLT3RITD (+/− TKD) mutations according to treatment
Fig S2 Distribution of patients with isolated FLT3R TKD mutations according to treatment