Abstract
Mineral bone disorder (MBD) is an important complication of chronic kidney disease (CKD). However, there are limited data on the pattern of MBD in Indian CKD population. The aim of this study was to describe spectrum of MBD in patients with CKD in our center. This was a hospital-based cross-sectional observational study. Patients with stage 4 and 5 CKD were included in this study. Those receiving calcium supplement, vitamin D or its analogues, and calcimimetic were excluded. Serum/plasma levels of creatinine, albumin, calcium, phosphate, total alkaline phosphatase (TAP), intact parathormone (iPTH), and 25-OH vitaminD (25-vitD) were measured. Radiological survey of bones was carried out in all cases, and echocardiography done in selected patients. Statistical analysis was done using Sigmaplot 10.0 software. A total of 150 patients (114 males, 36 females) were included in this study. Mean age was 45.67±16.96 years. CKD stage 4 and 5D were found in 26% (n=39) and 74% (n=111) of study population, respectively. The most common underlying native kidney diseases in patients of CKD 4 and 5D were diabetic nephropathy (41.03%) and CGN (41.44%), respectively. Median (first quartile, third quartile) values for serum levels of corrected calcium (cCa), phosphate, cCaXPO4 product, TAP, plasma iPTH, and 25-vitD in stage 4 CKD were 8.36 (7.79, 8.91) mg/dL, 4.9 (3.92, 6.4) mg/dL, 41.11 (34.01, 53.81) mg2/dL2, 97 (76.5, 184.25) IU/L, 231 (124.5, 430.75) pg/mL, and 12 (6.98, 23.55) ng/mL, respectively; and in stage 5D CKD were 8.36 (7.66, 8.95) mg/dL, 5.7 (4.23, 6.95) mg/dL, 46.5 (37.16, 54.47) mg2/dL2, 180 (114.5, 276.25) IU/L, 288 (169.75, 625.0) pg/mL, and 18.4 (10.0, 26.4) ng/mL, respectively. Prevalence of hypocalcemia (56.41% vs. 54.95%), hyperphosphatemia (64.10% vs. 70.27%), and hyperparathyroidism (84.62% vs. 88.29%) was not different between patients with CKD 4 and 5D. However, iPTH level outside the target range and increased TAP level were significantly (P<0.001) more common in CKD stage 5D. Multiple logistic regression analysis for hyperparathyroidism revealed significant inverse correlation with cCa in CKD 5D. There were no significant differences in vitamin D status and prevalence of valvular calcification between CKD stage 4 and 5D. X-ray revealed renal osteodystrophy in 8 (5.33%) patients, while it was normal in 118 (78.67%) patients. Secondary hyperparathyroidism, hyperphosphatemia, hypocalcemia, increased TAP, and 25-OH vitamin D deficiency and insufficiency were quite common in CKD 4 and 5 patients. The commonest type of MBD in CKD 4 and 5D was secondary hyperparathyroidism.
Keywords: Chronic kidney disease, hyperparathyroidism, hyperphosphatemia, hypocalcemia, mineral bone disorder
Introduction
Chronic kidney disease (CKD) is a global public health problem affecting 5–10% of world population.[1,2] As kidney function declines, there is progressive deterioration in mineral homeostasis manifesting as disruption of serum and tissue concentrations of phosphorus and calcium, as well as changes in circulating levels of hormones such as parathyroid hormone (PTH), 25-hydroxyvitamin D [25(OH)D], 1,25-dihydroxyvitamin D [ 1, 25(OH)2D], fibroblast growth factor-23 (FGF-23), and growth hormone. These mineral and endocrine functions are critically important in the regulation of both initial bone formation during growth (bone modeling) and bone structure and function during adulthood (bone remodeling). As a result, bone abnormalities are found almost universally in patients with CKD requiring dialysis (stage 5D), and in the majority of patients with CKD stages 3–5.[3] More recently, there has been an increasing concern of extra-skeletal calcification that may result from the deranged mineral and bone metabolism of CKD and from the therapies used to correct these abnormalities. Numerous cohort studies have shown associations between disorders of mineral metabolism and fractures, cardiovascular disease, and mortality in patients with CKD.[4–11] However, despite high prevalence of mineral bone disorders (MBDs) in CKD patients, there are limited data on MBD in Indian CKD patients. The aim of this work was to study the prevalence and pattern of MBDs in patients with CKD stage 4 and 5.
Materials and Methods
This prospective cross-sectional study was conducted in the department of Nephrology, Institute of Medical Sciences, Banaras Hindu University, India, between August 2008 and July 2010. Patients with CKD stage 4 and 5 attending the Department of Nephrology formed the subject load of this study. Patients of stage 4 and 5 CKD with the following characteristics were excluded from the study: (i) patients taking calcium supplement, phosphate binder, vitamin D or its active metabolites and analogues, calcimimetic, glucocorticoid, bisphosphonate, NSAIDs, phenytoin, or warfarin; (ii) patients having rheumatologic diseases such as rheumatoid arthritis and ankylosing spondylitis, or primary parathyroid hormone disorders; and (iii) those having liver disease or history of bone fracture in preceding 6 months. Institute ethical committee approved this work. CKD was defined and classified as per K/DOQI criteria.[3] The eGFR was calculated from serum creatinine level using the Cockcroft–Gault equation. The diagnosis of underlying basic kidney disease was made on clinical evidences.
CKD–MBD was defined as a systemic disorder of mineral and bone metabolism due to CKD manifested by either one or a combination of the following:[12,13] (i) abnormalities of calcium, phosphorus, PTH, or vitamin D metabolism; (ii) abnormalities in bone turnover, mineralization, volume, linear growth, or strength; or (iii) vascular or other soft-tissue calcification. The definitions for hypocalcemia (cCa<8.5 mg/dL), hypercalcemia (cCa>10.5 mg/dL), hyperphosphatemia (PO4>4.5 mg/dL), hypophosphatemia (PO4<2.5 mg/dL), elevated total alkaline phosphatase level (TAP>112 IU/L), hyperparathyroidism (iPTH>69 pg/mL), hypoparathyroidism (iPTH<10 pg/mL), vitamin D deficiency (<10 ng/mL), vitamin D insufficiency (10–30 ng/mL), and vitamin D sufficiency (>30 ng/mL) were same for CKD stages 4, 5, and 5D. However, target range for iPTH in CKD 4-5 and CKD 5D was different, namely 10–69 and 138–621 pg/mL (2–9 times the upper limit of normal for the assay), respectively.[13]
Serum creatinine, albumin, calcium, phosphate (PO4), TAP, hemoglobin, uric acid, and urinary protein excretion were measured using standard laboratory techniques. Plasma intact parathormone (iPTH) was measured using the solid phase, two-site chemiluminescent enzyme-labeled immunometric assay (Immulite/Immulite 1000). Plasma 25-OH vitamin D (25-vitD) assay was done using the equilibrium radioimmunometric assay (DiaSorin I125 RIA Kit). Radiological survey of bone was done with lateral X-ray of skull, dorsolumbar spine and abdomen, as well as anteroposterior views of pelvis and both wrists including hands. We looked specifically for changes of hyperparathyroidism, osteomalacia, osteoporosis, fracture, as well as soft tissue and vascular calcification. 2-D echocardiography was carried out to detect valvular calcification.
Statistical analysis
Statistical analysis was done using Sigmaplot 10.0 software. Various parameters of the whole group were analyzed as well as the parameters were compared between the two groups of CKD stages 4 and 5. Parametric variables were compared using unpaired t-test and Mann–Whitney Rank Sum test. Non-parametric variables were compared using Chi-square test. A P value<0.05 was taken as significant. Vitamin D levels between patients with and without hyperparathyroidism in both groups were compared as well as linear regression analysis between Vitamin D level and iPTH level in each group was done. Cox multiple logistic regression analysis was carried out to analyze the association of functional abnormality of parathyroid hormone with serum levels of calcium (corrected for albumin level), phosphate, TAP, and creatinine, as well as eGFR, duration of symptoms, and age of the patient. Different parameters of CKD-MBD were also compared between those with and without diabetes mellitus.
Results
One hundred and fifty patients (114 males, 36 females) were included in this study. Mean age was 45.67±16.96 (range 12–85) years. Demographic and clinical parameters of study population are given in Table 1. All patients of CKD stage 5 required dialysis at presentation and hence were essentially CKD stage 5D. Patients were divided into two groups: Group I included patients with CKD stage 4 and group II composed of patients with CKD stage 5D. Group I had 26% (n=39) and group II had 74% (n=111) of patients. The most common underlying native kidney disease [Table 1] in patients of CKD 4 was diabetic nephropathy (DN 41.03%) followed by chronic glomerulonephritis (CGN 20.51%), chronic interstitial nephritis (CIN 18.94%), and polycystic kidney disease (PKD 12.82%). In contrast, the most common underlying native kidney disease in patients of CKD 5D was CGN (41.44%) followed by DN (21.62%), CIN (15.31%), and obstructive uropathy (11.71%). Laboratory parameters and various mineral and bone disorders in patients with CKD stages 4 and 5D are given in Tables 2 and 3, respectively. The most common MBD in group I was hyperparathyroidism (84.62%) followed by hyperphosphatemia (64.10%), hypocalcemia (56.41%), and elevated TAP level (43.59%). In group II patients, the most common MBD was hyperparathyroidism (88.29%) followed by elevated TAP level (76.66%), hyperphosphatemia (70.27%), and hypocalcemia (54.95%). Prevalence of hypocalcemia, hyperphosphatemia, and hyperparathyroidism were quite high in both groups but was not significantly different between CKD 4 and 5D. However, increased TAP level and iPTH outside the target range were significantly more common in CKD stage 5D (P<0.001). Multiple logistic regression analysis was done for parathyroid functional status in two groups taking age of the patients, duration of symptoms, corrected serum calcium, serum phosphate, TAP, creatinine, and eGFR [Table 4]. No significant correlation was found in CKD 4. However, corrected serum calcium level was found to have significantly inverse correlation with hyperparathyroidism in CKD stage 5D (r –1.176, odds ratio 0.309, 95% CI of 0.133–0.733, P=0.008). Serum cCa also significantly correlated with iPTH levels outside the target range in CKD 5D. The odds ratio of cCa for iPTH levels above the target range was 0.495 (r –0.696, 95% CI of 0.30–0.83, P=0.007) and the odds ratio of cCa for iPTH levels below the target range was 4.577 (r +1.521, 95% CI of 1.984–10.559, P<0.001). There was no significant association between parathyroid functional status and other biochemical variables. Vitamin D deficiency and insufficiency were quite prevalent in both groups. However, there was no significant difference in vitamin D status between CKD stage 4 and 5D. The mean vitamin D levels in those with and without hyperparathyroidism in CKD stage 4 were 15.62±11.21 ng/mL and 20.88±14.39 ng/mL (P=0.431), respectively, and in CKD stage 5D were 18.19±11.19 ng/mL and 32.46±15.56 ng/mL (P=0.004), respectively. The linear regression analysis between iPTH and vitamin D levels revealed significant inverse correlation in CKD 5D (regression coefficient= –20.85, F=10.67, P=0.002) but not in CKD 4 (regression coefficient=–4.85, F=1.218, P=0.284). Different parameters of CKD-MBD were compared between those with and without diabetes mellitus in the whole study cohort [Table 5]. Patients with diabetes mellitus (n=46) had significantly lower serum phosphate (P=0.038), TAP (P<0.001), and iPTH (P=0.042) levels as compared with patients without diabetes mellitus (n=104). However, corrected serum calcium and vitamin D levels and cCaXPO4 product were not significantly different between those with and without diabetes. X-ray revealed features suggestive of renal osteodystrophy in 8 (5.33%) patients and other bony changes in 24 (16%) patients, while it was normal in 118 (78.67%) patients. No evidence of soft tissue or vascular calcification was found in any patient. 2-D echocardiography was done in 70 patients and it revealed valvular calcification in 25% and 46% of patients in group I and II, respectively (P=0.387). Multiple logistic regression analysis did not find correlation of valvular calcification with plasma levels of iPTH, corrected calcium, phosphate, or cCaXPO4 product.
Table 1.
Demographic and clinical parameters of patients with CKD 4 and 5D
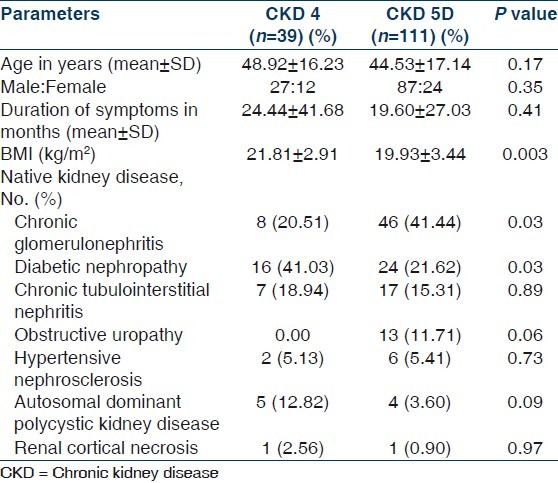
Table 2.
Laboratory parameters of patients with CKD 4 and 5D
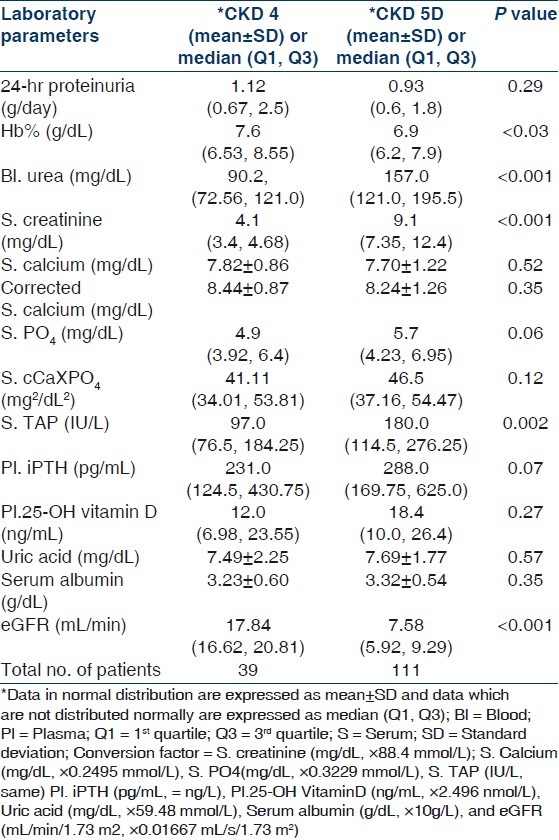
Table 3.
Mineral and bone disorders in patients with CKD 4 and 5D
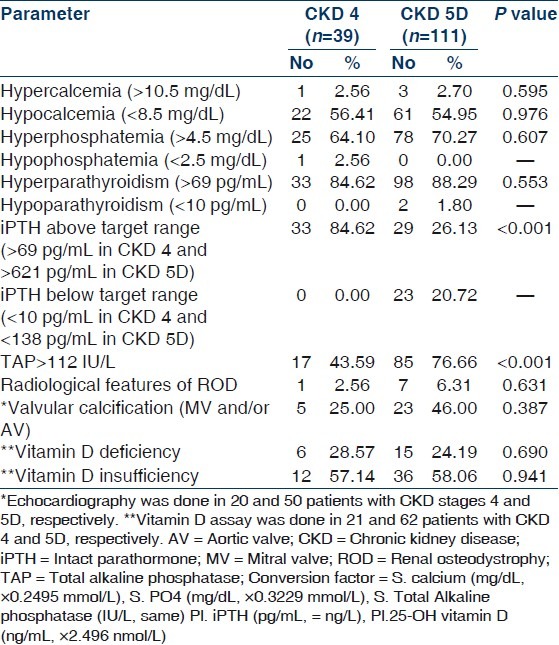
Table 4.
Multiple logistic regression analysis for parathyroid functional status in CKD stages 4 and 5D
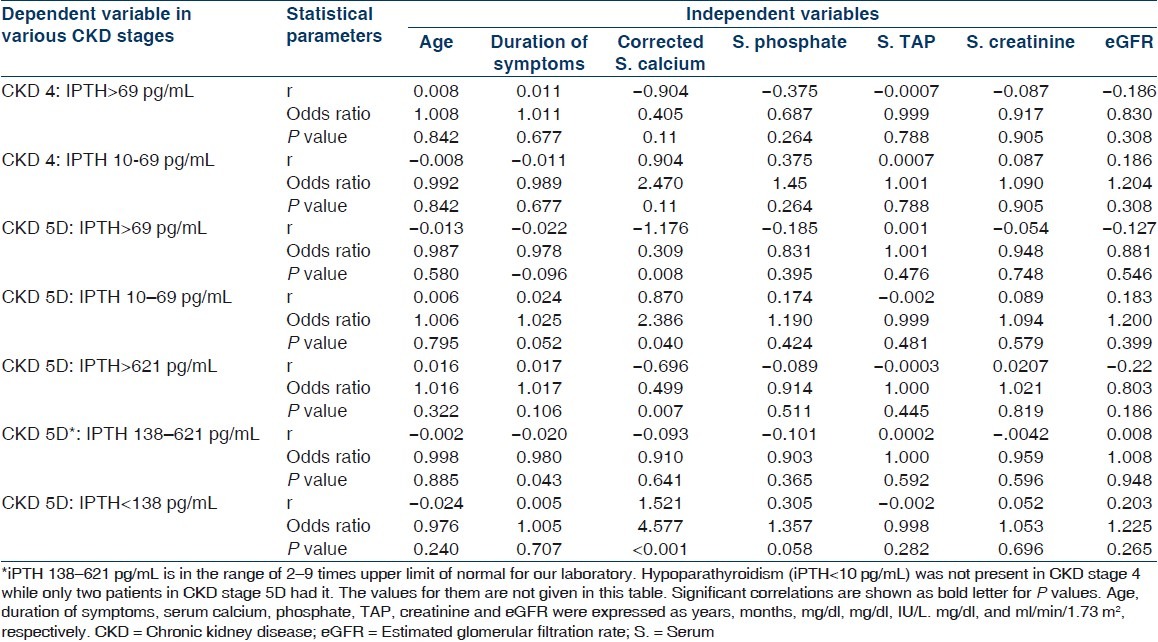
Table 5.
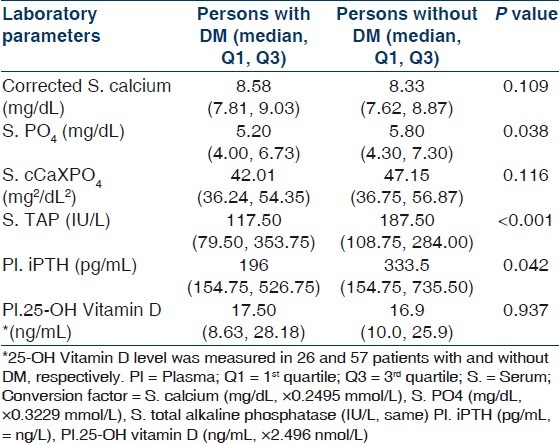
Comparison of different parameters of CKD-MBD in persons with (n=46) and without (n=104) diabetes mellitus in the whole study cohort including CKD stage 4 and 5D
Discussion
The mean age of our study population was similar to other studies.[14–18] However, higher mean age was reported in an Western and an Indian study.[19,20] We observed that males outnumbered females in both the groups (M:F 2.25:1 and 3.63:1 in groups I and II, respectively). There is male predominance among CKD population in most studies. In Nissenson's prevalence study from the United States, males had an overall prevalence of 1.6% and females 0.8%, this two-fold ratio was maintained at all levels of serum creatinine.[21] Among Indian studies, Agarwal et al.[20] (community based) showed a male prevalence of 48% among patients with serum creatinine more than 1.8 mg/dL, while other hospital-based studies found males constituting 60–78% of CKD population.[14–18] One of the main reasons for these differences in the age and gender of subjects may be that, in India, more males and younger persons visit hospitals than females and the elderly.
In our study, serum level of TAP was significantly higher (P=0.002) and hemoglobin was significantly lower (P=0.030) in patients of group II when compared with group I. Plasma iPTH level was higher in group II but it did not reach statistical significance. Mean serum levels of phosphate and uric acid were above the normal range, whereas those of calcium, albumin, and plasma 25-OH vitamin D were below the normal range in both groups of patients. However, there was no statistically significant difference of these values between the two groups. All patients were anemic with the median hemoglobin being 7.6 gm/dL. Agarwal showed that the mean corrected serum calcium in stage 4 and 5 CKD was 8.8 and 8.1 mg/dL, and the values for phosphate, TAP, and iPTH were 4.6 and 6.0 mg/dL, 256.8 and 245.3 IU/dL and 184.0 and 339.5 pg/mL, respectively.[14] Our study corroborated with the study of Agarwal, although the median iPTH in stage 4 and 5D CKD were, respectively, higher and lower in our patients. Jabbar, et al. found a mean iPTH of 308.8±177.5 pg/mL in patients with stage 4 and 5 CKD.[22] The median iPTH of patients with CKD stage 4 and 5 in our study was 279.75 pg/mL. Due to decreased GFR and altered endocrine function of kidneys, the parathyroid-vitamin D-renal axis gets deranged which results in phosphate retention, hypocalcemia, decreased active vitamin D, and secondary hyperparathyroidism in majority of patients.[23–27] 25-OH vitamin D deficiency is common in CKD stage 3–5. LaClair, et al. found 25-OH vitamin D level <75 nmol/L in 83% of patients with CKD stage 4.[27] In a study of Jabbar, et al., more than 90% of patients with CKD stage 4 and5 had vitamin D level less than 30 ng/mL.[28] In our study, 83.13% of patients with CKD stage 4 and 5D had vitamin D level less than 30 ng/mL. Moreover, in CKD stage 5D vitamin D level was significantly lower in those with hyperparathyroidism in comparison to those who did not have hyperparathyroidism. In fact, vitamin D level showed an inverse relationship to iPTH level in this group. Although supplementation of cholecalciferol may improve secondary hyperparathyroidism without the need for the active compound (calcitriol or other analogues), this issue has not been studied adequately till now.[29,30]
In this study, X-ray revealed features suggestive of renal osteodystrophy in only 5% patients and other bony changes in 16% patients while it was normal in the rest. Radiological studies aimed at detecting renal osteodystrophy by plain X-ray are sparse in recent times. The studies done in early 1970s found osteitis fibrosa in almost 50% of cases on dialysis.[31–33] However, Kaushal, et al. in 1989 reported osteitis fibrosa in 20% of patients on dialysis.[34] The prevalence of osteosclerosis varied widely from 0% to 54%.[33–36] Parfitt, et al. in 1977 observed osteomalacia in 20% of Australian patients with renal bone disease.[37] The prevalence of osteomalacia decreased following cessation of use of aluminium containing phosphate binders and better water treatment for hemodialysis. We found very low prevalence of radiological abnormalities in our patients which is consistent with the trend of lower prevalence in more recent studies.[34] The conventional X-ray is an insensitive tool to identify renal bone disease. Indeed, the recent KDIGO guidelines did not advocate plain X-ray for the evaluation of bone disease in CKD and even recommended restricted use of DEXA for measuring bone mineral density.[13] However, KDIGO guidelines recommended use of X-ray for the detection of vascular calcification. The exact bone disease can only reliably be known by histomorphometric study which again needs judicious use as recommended by KDIGO.
MBDs are well described in patients with CKD.[23–27,38–40] However, only few studies addressed these disorders in Indian patients.[14,22,37] Agarwal described hypocalcemia in 29.9% and 49.6% in CKD stage 4 and 5, respectively, and hyperphosphatemia in 45% and 41.8%, respectively.[14] LaClair, et al. found hypocalcemia (Ca<8.5 mg/dL) in 8% and 28%, and hyperphosphatemia (PO4>4.5 mg/dL) in 20% and 50% of patients of CKD stages 4 and 5, respectively.[27] Thus, the western data showed a lower prevalence of hypocalcemia and hyperphosphatemia (except hyperphosphatemia in stage 5) in comparison to the data of Agarwal, et al. from India.[14,27] Moreover, we found much higher prevalence of hypocalcemia and hyperphosphatemia even in comparison to the findings by Agarwal. Agarwal found hyperparathyroidism in 57.8% of patients with CKD stage 4 and in 39.4% of patients with CKD stage 5.[14] He defined hyperparathyroidism (iPTH>110 pg/mL in stage 4 and iPTH>300 pg/mL in stage 5 CKD) using K/DOQI guidelines.[3] Jabbar, et al. observed prevalence of hyperparathyroidism in 60% of their patients of CKD stage 4 and 5 taking a cutoff of iPTH>300 pg/mL for both stages.[22] La Clair, et al. found that 31% of CKD stage 4 patients had iPTH>110 pg/mL.[27] In our study, the prevalence of hyperparathyroidsm was much higher in both CKD stage 4 and 5D in comparison to previous studies. However, the prevalence of iPTH above the target range was lower in stage 5D in our study. This may be due to the use of different definitions and cutoff values for target range of iPTH. The cutoff in our study was 69 pg/mL (i.e., the upper limit of normal for the laboratory assay) for stage 4 CKD and 621 pg/mL (i.e., nine times the upper limit of normal for the laboratory assay) for stage 5D as recommended by the recent KDIGO guidelines.[13] Thus, the lower cutoff value in CKD stage 4 and higher value for CKD stage 5D might have resulted in higher and lower prevalence of iPTH above the target range in stages 4 and 5D, respectively. Nevertheless, these were the patients who might have high turnover bone disease and needed aggressive treatment to suppress the parathyroid. Thus, the prevalence of patients with iPTH above target range in more than four-fifth and one-quarter of patients of CKD stage 4 and 5D, respectively, is of concern. TAP also signifies high turnover bone disease when elevated and interpreted in appropriate circumstances. In this study, TAP level >112 IU/L (i.e., upper level of normal) was present in 43.59% and 76.66% of patients of CKD stage 4 and 5D, respectively. It may suggest that many more patients in stage 5D might had high turnover bone disease than was suggested by the high cutoff level of iPTH in this group. This subgroup of patients could be identified by serial estimation of iPTH and TAP which would show an increasing trend in them. Indeed, KDIGO recommended that the treatment of MBD be based on trend in changes of biochemical parameters rather than on abnormalities at a single point of time.[13] Jabbar, et al. found raised bone alkaline phosphatase (>45 IU/L) in 60% of their stage 4 and 5 CKD patients.[22]
In our study, among patients with CKD 5D, 30.43% of patients with iPTH below target range were diabetic. In contrast, only 10.34% of patients with iPTH above target range had diabetes. Moreover, in the whole study cohort (n=150), patients with diabetes mellitus had significantly lower serum phosphate (P=0.038), TAP (P<0.001), and iPTH (P=0.042) levels when compared with patients without diabetes mellitus. They also had higher corrected calcium level but that did not reach statistical significance. This could mean that diabetic patients were more likely to have adynamic bone disease. Considering the fact that the low phosphate level might be due to poor nutrition and that these patients were not on any calcium containing medications, treatment of such patients would require cautious approach. Adynamic bone disease has been described to be more prevalent in diabetic patients especially in elderly or those on CAPD.[40] Hypoparathyroidism can be associated with adynamic bone disease. However, no such association of adynamic bone disease with diabetes mellitus could be conclusively found in our study as we did not study the bone histology.
Multiple logistic regression analysis revealed strong correlation of serum level of corrected calcium with both hyperparathyroidism and iPTH outside the target range in patients with CKD stage 5D. Agarwal also found hypocalcemia to be associated with hyperparathyroidism.[14] In his study, multivariate logistic regression analysis taking age of patient, stage of CKD, hypocalcemia, and hyperphosphatemia, the odds ratio of developing hyperparathyroidism with hypocalecemia was 2.8 (95% CI 1.4–4.9, P<0.001), while other factors were not significantly associated.
In summary, MBDs such as secondary hyperparathyroidism, hyperphosphatemia, hypocalcemia, and vitamin D deficiency and insufficiency were quite prevalent both in CKD stage 4 and in incident-dialysis patients. Serum level of cCa was significantly correlated with iPTH level outside the target range in patients with advanced CKD (stage 5D).
Limitations of the study
We did not carry out bone biopsy to assess the abnormalities in bone turnover, mineralization, volume, linear growth, or strength. The categorization of bone disease in the absence of bone biopsy remains presumptive at the best. Hence, describing biochemical aspect of MBDs in the context of bone biopsy findings would be more desirable. Nonetheless, studies have shown biochemical parameters to correlate well with the bone histology and this study gives an overview of what we could expect in our day to day clinical practice.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, et al. The burden of kidney disease: Improving global outcomes. Kidney Int. 2004;66:1310–4. doi: 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Eustace JA. Epidemiology of Kidney Disease. In: Brennaer BM, editor. Brenner and Rector's The Kidney. 8th ed. Philadelphia: Saunders-Elsevier; 2008. pp. 615–32. [Google Scholar]
- 3.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 4.Piraino B, Chen T, Cooperstein L, Segre G, Puschett J. Fractures and vertebral bone mineral density in patients with renal osteodystrophy. Clin Nephrol. 1988;30:57–62. [PubMed] [Google Scholar]
- 5.Araújo SM, Ambrosoni P, Lobão RR, Caorsi H, Moysés RM, Barreto FC, et al. The renal osteodystrophy pattern in Brazil and Uruguay: An overview. Kidney Int Suppl. 2003;85:S54–6. doi: 10.1046/j.1523-1755.63.s85.13.x. [DOI] [PubMed] [Google Scholar]
- 6.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47:149–56. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 7.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–51. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 8.London GM, Marchais SJ, Guérin AP, Boutouyrie P, Métivier F, de Vernejoul MC. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol. 2008;19:1827–35. doi: 10.1681/ASN.2007050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taal MW, Roe S, Masud T, Green D, Porter C, Cassidy MJ. Total hip bone mass predicts survival in chronic hemodialysis patients. Kidney Int. 2003;63:1116–20. doi: 10.1046/j.1523-1755.2003.00837.x. [DOI] [PubMed] [Google Scholar]
- 10.Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol. 2007;18:2401–7. doi: 10.1681/ASN.2007010022. [DOI] [PubMed] [Google Scholar]
- 11.Blayney MJ, Pisoni RL, Bragg-Gresham JL, Bommer J, Piera L, Saito A, et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74:655–63. doi: 10.1038/ki.2008.248. [DOI] [PubMed] [Google Scholar]
- 12.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–53. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD) Kidney Int Suppl. 2009;76(113):S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal SK. Assessment of renal bone mineral disorder in naïve CKD patients: A single center prospective study. Indian J Nephrol. 2007;17:96. [Google Scholar]
- 15.Agarwal SK, Dash SC. Spectrum of renal diseases in India in adults. J Assoc Physicians India. 2000;48:594–600. [PubMed] [Google Scholar]
- 16.Mittal S, Kher V, Gulati S, Agarwal LK, Arora P. Chronic renal failure in India. Ren Fail. 1997;19:763–70. doi: 10.3109/08860229709037216. [DOI] [PubMed] [Google Scholar]
- 17.Sakuja V, Jha V, Ghosh AK, Ahmed S, Saha TK. Chronic renal failure in India. Nephrol Dial Transplant. 1994;9:871–2. [PubMed] [Google Scholar]
- 18.Mani MK. Chronic renal failure in India. Nephrol Dial Transplant. 1993;8:684–9. doi: 10.1093/ndt/8.8.684. [DOI] [PubMed] [Google Scholar]
- 19.McClellan WM, Port FK. Epidemiology of Chronic Kidney Disease. In: Molony DA, Craig JC, editors. Evidence-Based Nephrology. Oxford: Wiley-Blackwell; 2009. pp. 3–17. [Google Scholar]
- 20.Agarwal SK, Dash SC, Irshad M, Raju S, Singh R, Pandey RM. Prevalence of chronic renal failure in adults in Delhi, India. Nephrol Dial Transplant. 2005;20:1638–42. doi: 10.1093/ndt/gfh855. [DOI] [PubMed] [Google Scholar]
- 21.Nissenson AR, Pereira BJ, Collins AJ, Steinberg EP. Prevalence and characteristics of individuals with chronic kidney disease in a large health maintenance organization. Am J Kid Dis. 2001;37:1177–83. doi: 10.1053/ajkd.2001.24520. [DOI] [PubMed] [Google Scholar]
- 22.Jabbar Z, Aggarwal PK, Chandel N, Khandelwal N, Sakhuja V, Jha V. Noninvasive assessment of bone mineral status in indian CKD population-a cross-sectional study. Indian J Nephrol. 2007;17:93. doi: 10.4103/0971-4065.111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–30. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Huan J, Olgaard K, Nielsen LB, Lewin E. Parathyroid hormone 7-84 induces hypocalcemia and inhibits the parathyroid hormone 1-84 secretory response to hypocalcemia in rats with intact parathyroid glands. J Am Soc Nephrol. 2006;17:1923–30. doi: 10.1681/ASN.2005101136. [DOI] [PubMed] [Google Scholar]
- 25.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–201. [PubMed] [Google Scholar]
- 26.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 27.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, et al. Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–33. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Jabbar Z, Aggarwal PK, Chandel N, Sakhuja V, Jha V. Vitamin D deficiency in Indian CKD patients. Indian J Nephrol. 2007;17:95. doi: 10.4103/0971-4065.111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27:36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 30.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–9. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 31.Katz AI, Hamper CL, Merrill JP. Secondary hyperparathyroidism and renal osteodystrophy in chronic renal failure. Analysis of 195 patients, with observations on the effects of chronic dialysis, kidney transplantation and subtotal parathyroidectomy. Medicine (Baltimore) 1969;48:333–74. [PubMed] [Google Scholar]
- 32.Eastwood JB, Bordier PJ, de Wardne HE. Some biochemical, histological, radiological and clinical features of renal osteodystrophy. Kidney Int. 1973;4:128–40. doi: 10.1038/ki.1973.91. [DOI] [PubMed] [Google Scholar]
- 33.Meema HE, Rabinovich S, Meema S, Lloyd GJ, Oreopoulos DG. Improved radiological diagnosis of azotemic osteodystrophy. Radiology. 1972;102:1–10. doi: 10.1148/102.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Kaushal S, Rizvi SN, Bhardwaj OP. Macroradiography in patients of chronic renal failure. Ind J Radiol. 1989;43:345–9. [Google Scholar]
- 35.Cohen ME, Cohen GF, Ahad V, Kaye M. Renal osteodystrophy in patients on chronic hemodialysis: A radiological study. Clin Radiol. 1970;21:124–34. doi: 10.1016/s0009-9260(70)80099-x. [DOI] [PubMed] [Google Scholar]
- 36.Johnson C, Graham CB, Kings F, Curtis B. Roentgenographic manifestations of chronic renal disease treated by periodic hemodialysis. Am J Roentgenol Radium Ther Nucl Med. 1967;101:915. doi: 10.2214/ajr.101.4.915. [DOI] [PubMed] [Google Scholar]
- 37.Parfitt AM. Clinical and radiographic manifestations of renal osteodystrophy. In: David DS, editor. Calcium metabolism in renal failure and nephrolithiasis. New York: John Willey and Sons; 1977. p. 150. [Google Scholar]
- 38.Gerakis A, Hadjidakis D, Kokkinakis E, Apostolou T, Raptis S, Billis A. Correlation of bone mineral density with the histological findings of renal osteodystrophy in patients on hemodialysis. J Nephrol. 2000;13:437–43. [PubMed] [Google Scholar]
- 39.Lindergård B, Johnell O, Nilsson BE, Wiklund PE. Studies of bone morphology, bone densitometry, and laboratory data in patients on maintenance hemodialysis treatment. Nephron. 1985;39:122–9. doi: 10.1159/000183355. [DOI] [PubMed] [Google Scholar]
- 40.Moe SM, Sprague SM. Mineral Bone Disorders in Chronic Kidney Disease. In: Brennaer BM, editor. Brenner and Rector's The Kidney. 8th ed. Philadelphia: Saunders-Elsevier; 2008. pp. 1784–814. [Google Scholar]


