Table 1.
Screening of Various Vinylcyclopropanes
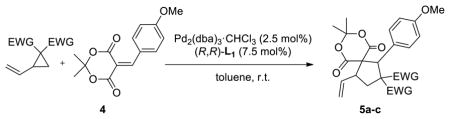
| |||
|---|---|---|---|
| entry | substrate | product | |
| 1 |
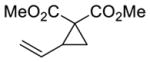 1 |
|
5a 97%a, 1.5:1 d.r.b 39% e.e. (major)c 72% e.e. (minor)c |
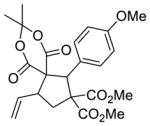
| 2 |
 6 |
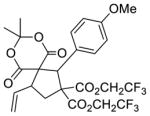
|
5b 84%a, 2.1:1 d.r.b 29% e.e. (major)c 79% e.e. (minor)c |
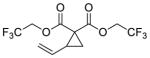
| 3 |
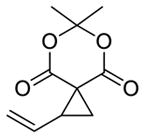 7 |
|
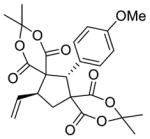
5c 22%a, >20:1 d.r.b 94% e.e. (major)c |
Isolated yield.
Diastereomeric ratio (d.r.) determined by 1H NMR.
Enantiomeric excess (e.e.) of major and minor diastereomer, respectively, determined by chiral HPLC.
