Table 3.
Cycloaddition with Meldrum’s Acid Alkylidenes
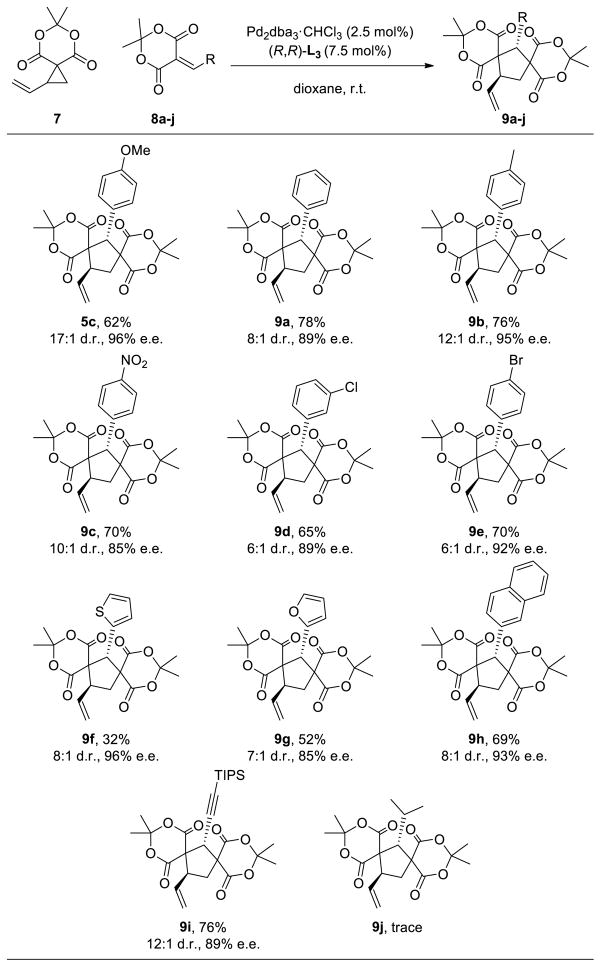
|
Yields given are of the isolated products. Diastereomeric ratios (d.r.) determined by 1H NMR. Enantiomeric excesses (e.e.) of the major diastereomer determined by chiral HPLC.
Cycloaddition with Meldrum’s Acid Alkylidenes

|
Yields given are of the isolated products. Diastereomeric ratios (d.r.) determined by 1H NMR. Enantiomeric excesses (e.e.) of the major diastereomer determined by chiral HPLC.