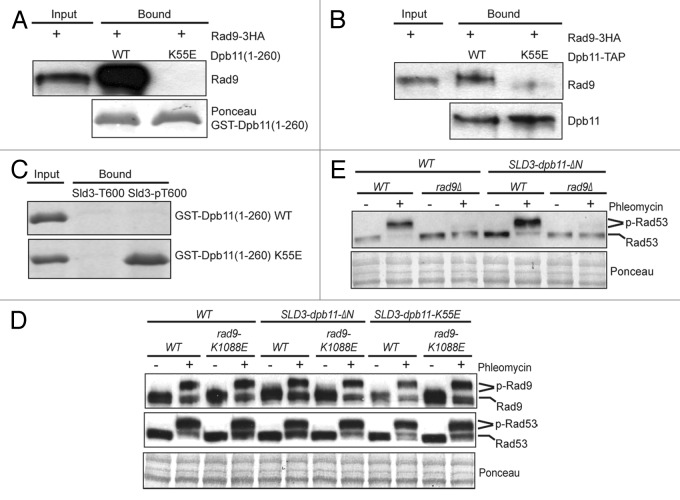
Figure 1. The interaction between Rad9 and the N-terminal BRCT domains of Dpb11 is dispensable for DNA damage checkpoint activation. (A) Recombinant GST-Dpb11(1–260) (WT or K55E) was bound to glutathione beads and used to bind to Rad9–3HA in cell lysates derived from G2/M-arrested cells. Bound protein was detected with anti-HA antibody. (B) Effect of the K55E mutation of full-length Dpb11-TAP on its binding to endogenous Rad9–3HA from G2/M-arrested cells. The TAP tag consists of a 6xHIS-3xFLAG-Protein-A tag.24 Dpb11-TAP was first bound to the IgG beads and then used to bind to Rad9. (C) An N-terminally biotinylated Sld3 peptide corresponding to amino acids 588–612 (RVDSEENVQVQAT600PAVKKRTVTPNK) with the T600 residue unphosphorylated or phosphorylated was immobilized on straptavidin beads and used to pull-down recombinant GST-Dpb11(1–260) fragments (WT or K55E mutant). Bound proteins were detected by Coomassie blue staining. (D) Western blot analysis of Rad9–3HA and Rad53 gel mobility shift in G2/M-arrested WT, SLD3-dpb11-ΔN and SLD3-dpb11-K55E cells with or without rad9-K1088E. Cells were treated with or without phleomycin. Ponceau stain was used as a loading control. SLD3-dpb11-ΔN has an N-terminal truncated Dpb11 (amino acids 253–764). (E) western blot analysis of Rad53 gel mobility shift in G2/M-arrested WT, rad9Δ, SLD3-dpb11-ΔN and SLD3-dpb11-ΔN rad9Δ cells with or without phleomycin treatment.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.