Abstract
Predators may affect prey population growth and community diversity through density mediated lethal and trait mediated non-lethal effects that influence phenotypic traits of prey. We tested experimentally the roles of thinning the density of prey (lethality) in the absence of predator cues and density and trait mediated effects (lethality + intimidation) of predatory midge Corethrella appendiculata on competing native and invasive mosquito prey. Predator-mediated reductions in prey and density reductions in the absence of C. appendiculata resulted in lower percent survivorship to adulthood and estimates of the finite rate of increase (λ′) for invasive mosquito Aedes albopictus relative to that of controls. In most instances, thinning the density of prey in the absence, but not in the presence, of C. appendiculata cues resulted in lower survivorship to adulthood and λ′ for native mosquito Aedes triseriatus relative to that of controls. Together, these results suggested trait mediated effects of C. appendiculata specific to each species of mosquito prey. Release from intraspecific competition attributable to density reductions in the absence, but not in the presence, of C. appendiculata enhanced growth and lengthened adult lifespan relative to that of controls for A. albopictus but not A. triseriatus. These results show the importance of predator-mediated density and trait mediated effects on phenotypic traits and populations of invasive and native mosquitoes. Species-specific differences in the phenotypic responses of prey may be due, in part, to longer evolutionary history of C. appendiculata with A. triseriatus than A. albopictus.
Introduction
Predators can have a major influence on prey population growth and community diversity [1]–[3]. The effect of predators on prey may largely be influenced by underlying environmental context such as the presence of other competing species [4], chemical contaminants [5]–[7], and habitat complexity [8], [9]. The most obvious effects of predators are attributable to prey capture and consumption, a direct lethal (density mediated) effect. However, the presence of predators may have non-lethal (trait mediated) effects attributable to intimidation that alter phenotypic traits of prey [10]–[12] and even extend to non-prey populations [13]. Non-lethal effects of predators may include inducing changes in behavior, development, growth, morphology and physiology [10], [14], [15]. Predator-induced changes in phenotypic traits of prey are often defensive strategies [16]–[17]. For instance, predator cues induce development of defensive spines in Daphnia [16], alterations in shell morphology in snails [18], heavier exoskeletons and longer caudal filaments in mayflies [19], longer caudal spines in dragonflies [20], and reduced activity and use of refugia in amphibians, insects, and other animals [14], [21]. A meta-analysis showed that the relative magnitude of non-lethal effects of predation may exceed those effects attributable to lethality [17]. Identification of non-lethal effects of predators (and measurement of their relative magnitude) is often difficult, especially when their effect is directionally similar to lethal effects of predators.
Modification of prey traits due to the presence of predators, either through lethal or non-lethal pathways, is often assumed to arise via phenotypic plasticity. However, alterations in prey traits may arise due to other processes that change phenotypes, which might include selection among individuals of different phenotypes [22]. For instance, a predator may alter the distribution of prey traits in a population via selective removal of some prey phenotypes, as demonstrated theoretically [23]–[24] and empirically [25]. Plastic responses may be reversible within an individual's life time (e.g., behavioral changes) whereas selective effects occur across generations if the traits are heritable.
Mosquitoes inhabiting water-holding containers (phytotelmata, tires, cisterns) are a tractable model system to investigate lethal and non-lethal effects of predators on phenotypic traits of prey [4], [26]. Water-filled containers are relatively simple communities that are occupied by numerous mosquito species [27]. Biotic interactions such as predation and competition are relatively common and shape mosquito communities [4] and may impact adult traits related to transmission of pathogens. For instance, intra and interspecific competition alter individual life history traits, population performance, and vector competence for arthropod-borne viruses e.g., dengue virus [28], LaCrosse virus [29], and Sindbis virus [30]–[31]. The presence of predators, as well as predator cues in the absence of capture and consumption, alters behavior and life history traits of mosquitoes including Culex pipiens molestus (Forskal) [32], Aedes triseriatus (Say) [33]–[35], and Aedes albopictus (Skuse) [34]–[35].
The Asian tiger mosquito A. albopictus (Skuse) is an invasive mosquito which has expanded is geographic range throughout much of the world in recent decades [36]. The global spread of A. albopictus is a public health concern since it can be a vector of numerous arthropod-borne (arbo) viruses and has been implicated as the vector responsible for recent outbreaks of dengue and chikungunya viruses. Range expansion of A. albopictus has been associated with declines in the abundance of the yellow fever mosquito Aedes aegypti (L.) in southeastern USA and in the Bermuda Islands [37], most likely attributable to the former's competitive superiority [26], [36]–[38].
Declines in the native Eastern treehole mosquito A. triseriatus have not been associated with establishments of invasive A. albopictus [39]–[40]. Aedes albopictus and A. triseriatus share similar container habitats (e.g., treeholes), some of which are also commonly occupied by predatory larvae of the mosquito Toxorhynchites rutilus (Coquillett) and the midge Corethrella appendiculata (Grabham). These Aedes species detect predators by water-borne and physical cues [33]–[34], [41]–[42] and potential trade-offs between competitive ability and vulnerability to predation may affect coexistence [43]–[46]. Some studies have demonstrated that A. albopictus is competitively superior to A. triseriatus in the absence of predators [47]–[51]. Laboratory and field studies have demonstrated that these species differ in their vulnerability to predation [33]–[34], [43]–[44]. Larger size of A. triseriatus and greater adoption than A. albopictus of low risk behaviors contributes to lower vulnerability of the native species to predation by C. appendiculata [34], [45], relationships found in other systems where prey traits contribute to resistance to predation [14], [52]. Both A. triseriatus and A. albopictus may grow to a body size relatively invulnerable to size-dependent predation by C. appendiculata [34], [42]. However, intra and interspecific competition may lead to food limitation which slows growth rates and lengthens exposure to predation. Empirical and theoretical studies have demonstrated that the presence of C. appendiculata contributes to invasion resistance and coexistence of the competing mosquito prey species [45]–[46]. However, the relative importance of thinning the density of prey and of trait mediated effects on the outcome of competition between A. triseriatus and A. albopictus has not been determined. Nonconsumptive effects of predators have been shown to reverse the outcome of competition in other systems [53]–[54] and may also have demographic consequences (e.g., growth and development) among container mosquitoes. Here we investigate these ideas in the context of competing A. triseriatus and A. albopictus and the predator C. appendiculata.
Methods and Materials
Predator, prey and experimental design
The experiment included a two-species prey community with two levels of larval treatment (intra and interspecific competition) for each prey species crossed with three levels of predation with four replicates (2×3 factorial design). Predator treatments consisted of predator absent (control), predators present and prey removal. The latter predator treatment simulated the depletion of prey by predation by removing mosquito larvae from containers according to a daily mortality schedule. The experimental containers serving as replicates consisted of cylindrical translucent plastic containers (16 cm height, 15 cm diameter) and were set up five days before the addition of mosquito larvae. Each container received 2.0 L tap water and 4.0 g Quercus virginiana (live oak) leaves and four days later we added 0.2 g of an equal mixture of brewer's yeast and lactalbumin. Each container was assigned a larval prey treatment and received first instar Florida-derived A. triseriatus and A. albopictus as 100∶0, 50∶50, and 0∶100, respectively. Aedes albopictus (generation F4) and A. triseriatus (generation > F20) were progeny from a colony collected as larvae from tires and treeholes in Indian River County, FL. These larval prey treatments represent intra and interspecific competition. An equal mixture of brewer's yeast and lactalbumin supplemental food (0.1 g) was added on days 6, 11, and 14 after addition of larvae. Experimental treatments were maintained at 24.2±0.5°C, 83.3±7.0% humidity and a 14∶10 hour light:dark photoperiod.
Predator present treatments consisted of the addition of four 4th instar C. appendiculata to each experimental container. Larvae of C. appendiculata were obtained from a Florida colony established from 200–300 individuals collected from water-filled containers (treeholes, tires). Rearing of C. appendiculata used methods described in [55]. Mosquito larvae in predator present treatments were exposed to non-lethal predator cues (trait mediated effects) as well as capture and consumption (density mediated effects) by C. appendiculata. Predator absent (control) treatments lacked the addition of C. appendiculata and so mosquito larvae only experienced intra and interspecific competition. We simulated the thinning effect of predation according to daily mortality schedules using a prey removal treatment. Thus, we exclude trait mediated effects attributable to the presence of C. appendiculata (e.g., changes in prey behavior). Every day the entire contents (water + larvae) of all experimental containers were emptied into enamel pans, subjected to treatment manipulation, and then returned to their original containers. Treatment manipulations included counting prey mosquitoes, removal of prey (i.e., in removal treatments), and maintaining C. appendiculata density the same from day to day (i.e., in the predator present treatments). For predator present treatments we enumerated mosquito larvae and removed and replaced dead C. appendiculata or pupae with 4th instar C. appendiculata. Enumerating mosquito larvae was the basis for creating daily mortality schedules. No attempt was made to identify the species of mosquitoes before emergence to adulthood. Each prey removal treatment replicate was paired with a predator present treatment replicate for the duration of the experiment. Every day equal numbers of larvae were removed from prey removal treatments based on the daily estimated mortality from paired predator treatments. A numbered grid consisting of 4×4 cm cells was drawn on the bottom of enamel pans used for holding the removal treatment contents. A randomly chosen number representing one of the cells was used to identify a cell to remove larvae. All larvae were removed from one cell before another random number was identified and larvae were removed from the second chosen cell. This process was repeated until the desired numbers of larvae were removed from each prey removal treatment replicate. The methodology used for removal treatments minimize the possibility for selective removal of one mosquito species over the other. Thus, we isolated the effects of density manipulation but did not account for species-specific prey selection in treatments where both species were present. The prey removal treatment simulated the thinning effect of predation by C. appendiculata (lethality) without associated trait mediated effects (e.g., prey behavioral modification) that may occur in the presence of C. appendiculata. The predator present treatment includes both density and trait mediated effects of C. appendiculata on mosquito prey (lethality + intimidation). In most natural situations, both density and trait mediated effects of predation occur simultaneously which was our reasoning for use of this latter treatment. Thus, we did not assess perception of predation risk independently from density-mediated effects.
Data measurements
Mosquito pupae from experimental containers were collected daily and transferred to plastic vials for adult emergence. Because pupae were transferred from the experimental containers to vials, mosquitoes were exposed to predators for only part of the time during their pupal stage. Newly emerged adult mosquitoes were scored by species and sex and the females were transferred daily to 0.5 L paperboard cages (1–5 females/cage) with mesh screening with continuous access to water-soaked cotton. Adult females were monitored for survivorship daily between 0900 and 1500 hours during the light period of the 24 hour daily cycle. Dead adults were recorded and stored at −20°C. We gauged treatment effects on mosquito prey by measuring percent survivorship to adulthood from the initial cohort of larvae, development time to adulthood, female dry weight and adult female lifespan. An estimated finite rate of increase (λ′) was calculated for each treatment replicate:
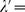 exp
exp
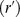


 |
where No is the original number of females in a cohort (assumed to be 50%), Ax is the number of females emerging to adulthood on day x, wx is the mean adult female size on day x, and f(wx ) describes the relationship between female size and the number of eggs produced. Masses of adult A. triseriatus and A. albopictus were determined by measuring dry weights (dried at 80°C for >48 h) on an electrobalance. D is the number of days from adult female emergence to oviposition. D is set at 12 days for A. triseriatus and 14 days for A. albopictus [47], [56]. We used the following fecundity-size relationships [f(wx )]:
A. triseriatus
A. albopictus
Statistical analyses
Treatment effects on mosquito responses were analyzed separately for A. triseriatus and A. albopictus. Treatment effects on mosquito survivorship, development time (female, male), and weight of adult females were analyzed using multivariate analysis of variance (MANOVA). Standardized canonical coefficients were used to determine the relative contribution of each response variable to significant MANOVA effects. Treatment effects on mosquito λ′ were analyzed using analysis of variance. When significant effects were detected, we used pairwise contrasts of means adjusting α = 0.05 for multiple comparisons (Tukey-Kramer adjustment for multiple comparisons, PROC GLM, SAS 9.22). Treatment effects on survival probability of adults, using lifespan of adult females, were compared using non-parametric survival analysis (PROC LIFETEST, SAS 9.22). Follow-up procedures used logrank test statistics to compare pairwise estimates of the survival probability functions adjusting for multiple comparisons using the Sidak method [58]. Adult lifespan was measured for 334 and 193 female adults for A. albopictus and A. triseriatus, respectively.
Results
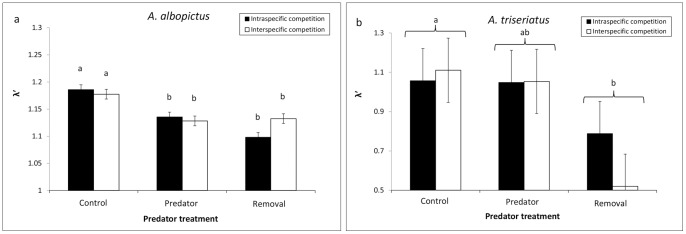
Analysis of variance demonstrated significant effects of the predator treatment on λ′ of both A. albopictus and A. triseriatus. Also, there was an interaction between the predator and intra and interspecific treatments on λ′ of A. albopictus (Table 1). Aedes albopictus in the control treatments had significantly higher λ′ than in predator present and prey removal treatments (Figure 1a). Despite indication of a significant interaction, after correcting for multiple comparisons, the intra and interspecific treatments had similar effects on λ′ of A. albopictus (Figure 1a). Aedes triseriatus λ′ in control treatments was significantly higher than in prey removal but not in predator present treatments (Figure 1b). Differences between the predator present and prey removal treatments were not signficant for A. triseriatus but trends (P = 0.06) showed higher λ′ in the predator present than the removal treatments (Figure 1b).
Table 1. Analysis of variance results for λ′′ of A. triseriatus and A. albopictus in response to treatment effects of predation, intra and interspecific competition, and their interaction.
| A. triseriatus | A. albopictus | |||||
| Source | d.f. | F | P | d.f. | F | P |
| Predation (P) | 2 | 4.28 | 0.0301 | 2 | 29.87 | <0.0001 |
| Competition (C) | 1 | 0.27 | 0.6075 | 1 | 0.72 | 0.4080 |
| P × C | 2 | 0.56 | 0.5798 | 2 | 3.74 | 0.0440 |
| Error | 18 | 18 | ||||
Significant effects are shown in boldface.
Figure 1. Estimated finite rate of increase (λ').
λ′ of a) A. albopictus and b) A. triseriatus for a significant interaction between predation and intra and interspecific competition for A. albopictus and a significant effect of predation treatment for A. triseriatus. Treatments followed by different letters are significantly different (P≤0.05) from one another. Brackets denote significant differences between treatments that include the cumulative effect of two mean values.
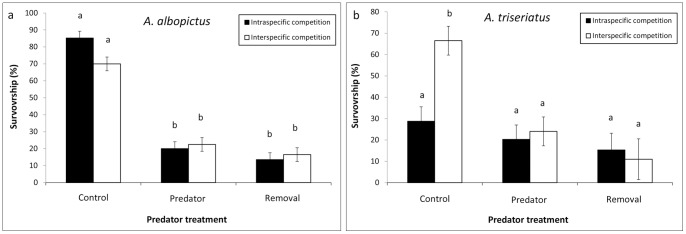
Multivariate analysis of variance showed a significant predator treatment effect on A. albopictus (Table 2). The magnitudes of the standardized canonical coefficients indicated that differences in percent survivorship to adulthood followed development time of females contributed the most to the significant predator effect (Table 2). Differences in development times of males and dry masses of females contributed to a lesser extent to the significant predator effect (Table 2). Aedes albopictus in the control treatments had significantly higher survivorship to adulthood than in the predator present and prey removal treatments (Figure 2a). Development time of females, but not males, were significantly longer in the control treatments than in the predator present and prey removal treatments (mean±S.E.; control, 10.15±0.08; predator present, 9.20±0.08; prey removal, 9.28±0.08). Pairwise contrasts of means for the predator treatments showed that adults were significantly larger in the prey removal treatment relative to the control and predator present treatments (mean±S.E.; control, 0.382±0.005; predator present, 0.399±0.005; removal, 0.425±0.005). Masses of A. albopictus were not significantly different between the control and predator present treatments.
Table 2. Multivariate analysis of variance on survivorship, development (female, male), and weight of adult females in response to predation and intra and interspecific treatments.
| Standardized canonical coefficients (SCCs) | |||||||
| Source | Pillai's Trace | d.f | P | Survivorship | Female development | Male development | Female weight |
| A. triseriatus | |||||||
| Predation (P) | 0.96 | 8, 26 | 0.0163 | 2.61 | 0.45 | −0.96 | −1.34 |
| Competition (C) | 0.77 | 4, 12 | 0.0007 | −2.39 | −0.32 | 1.18 | 1.80 |
| P × C | 1.04 | 8, 26 | 0.0067 | −2.38 | 0.05 | 1.40 | 1.58 |
| A. albopictus | |||||||
| Predation (P) | 1.41 | 8, 32 | <0.0001 | 3.25 | 0.85 | −0.16 | −0.18 |
| Competition (C) | 0.30 | 4, 15 | 0.2211 | −1.41 | 2.01 | −0.41 | 0.35 |
| P × C | 0.63 | 8, 32 | 0.1032 | 1.39 | −1.12 | 0.85 | −0.91 |
Significant effects are shown in boldface.
Figure 2. Survivorship to adulthood.
Survivorship of a) A. albopictus and b) A. triseriatus for predation and intra and interspecific competition treatments. Treatments followed by different letters are significantly different (P≤0.05) from one another.
Multivariate analysis of variance showed significant predator treatment, intra and interspecific treatment and interaction effects on A. triseriatus (Table 2). Standardized canonical coefficients indicated that differences in percent survivorship to adulthood followed by dry masses of females and development time of males contributed the most to the significant interaction treatment effect. Differences in development time of females had only a minor contribution to the interaction effect (Table 2). The interspecific competition treatments for A. triseriatus had significantly greater percent survivorship to adulthood in the control than other treatments (Figure 2b). Pairwise comparisons of the means of predator x intra and interspecific competition treatments for development times and dry masses of A. triseriatus were not significant.
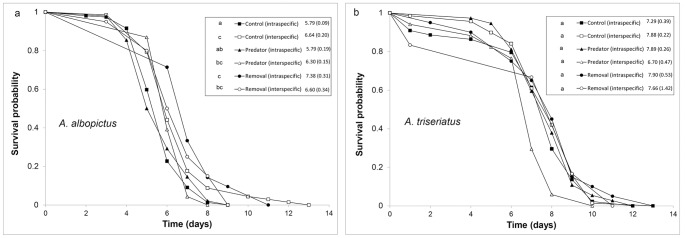
Non-parametric survival analyses demonstrated significant effects of the predator treatment, intra and interspecific treatment, and the interaction on survivor function estimates of A. albopictus females (Table 3). The survivor function is the probability that mosquitoes survive until time t. Comparison of survival distributions showed significantly steeper declines in survivor function estimates of adults from the control (intraspecific) treatments than all other treatments. However, the control (intraspecific) and predator (intraspecific) treatments were not significantly different from one another (Figure 3a). Pairwise contrasts of survivor functions for predator x intra and interspecific competition treatments showed steeper declines in survivor function estimates of adults from the predator (intraspecific) treatments than the prey removal (intraspecific) and control (interspecific) treatments (Figure 3a). Survival function estimates of A. triseriatus were not affected by the treatments (Table 3, Figure 3b).
Table 3. Non-parametric analysis for survival probability of adult A. triseriatus and A. albopictus females in response to treatment effects of predation, intra and interspecific competition, and their interaction.
| A. triseriatus | A. albopictus | |||||
| d.f. | ?2 | P | d.f. | ?2 | P | |
| Source | ||||||
| Predation (P) | 2 | 2.9278 | 0.2313 | 2 | 14.2592 | 0.0008 |
| Competition (C) | 1 | 0.0210 | 0.8847 | 1 | 11.8322 | 0.0006 |
| P × C | 5 | 9.115 | 0.1047 | 5 | 34.2433 | <0.0001 |
Significant effects are shown in boldface.
Figure 3. Survival of adult female mosquitoes.
Survival (proportion) probability of a) A. albopictus and b) A. triseriatus adult females. For Ae. albopictus there was a significant interaction between predator and intra and interspecific competition treatments. Treatments followed by different letters are significantly different (P≤0.05) from one another. Numbers in the figure legends indicate mean (± standard error) lifespans in days.
Discussion
To explore the relative roles of density mediated versus density and trait mediated effects on competing mosquitoes, we exposed competing A. triseriatus and A. albopictus to the thinning effect of predation without predators (lethality) as well as treatments where the predator C. appendiculata were present (lethality + intimidation). Previous studies have demonstrated that interspecific interactions between A. triseriatus and A. albopictus may be altered by the presence of C. appendiculata. Specifically, in the presence of C. appendiculata, the competitive advantage that A. albopictus has over A. triseriatus is diminished [43], [45]–[46]. In contrast, a meta-analysis showed competitive equivalence and no context dependence on competitive outcome between these two species [59]. Given that A. triseriatus is less vulnerable to predation by C. appendiculata than A. albopictus, we expected that A. triseriatus in interspecific treatments would perform worse in prey removal than in predator present treatments. However, neither the prey removal treatment nor the predator present treatment altered the outcome of interspecific interactions between A. triseriatus and A. albopictus, providing equivocal support for predator-mediated changes in competitive outcome. Interspecific competition is resource-dependent [60]–[61] and so nutrients may not have been sufficiently limiting to detect interspecific differences between A. triseriatus and A. albopictus. These results support a meta-analysis showing equivalent effects of inter- and intraspecific competition of A. triseriatus and A. albopictus [59].
Survivorship and λ′ were clearly altered by the presence of C. appendiculata and the thinning effect of prey in the absence of predators, but effects differed between prey species. The predator present and prey removal treatments reduced λ′ of A. albopictus relative to control treatments, suggesting that C. appendiculata effects were largely attributable to density and not trait mediated effects. In contrast, λ′ of A. triseriatus were similar in the presence of C. appendiculata and controls lacking predators. Therefore population growth of A. albopictus was reduced in the presence of C. appendiculata whereas population growth of A. triseriatus did not substantially differ from the controls. Greater effects of C. appendiculata on λ′ of A. albopictus may have been due to recent evolutionary contact with this predator whereas A. triseriatus has had longer evolutionary history with C. appendiculata. Variation in survival to adulthood contributes strongly to changes in λ′. For A. albopictus, the presence of C. appendiculata reduced survivorship by approximately 77% for intraspecific and 68% for interspecific treatments relative to the controls. A similar comparison for A. triseriatus showed a lower reduction of approximately 30% for intraspecific and 64% for interspecific treatments in percent survivorship. Our findings were consistent with other studies showing that A. triseriatus is less susceptible to capture and consumption by C. appendiculata than A. albopictus [34]. Regardless, estimates of λ′ were always greater than 1 for A. albopictus for all treatment groups, whereas some estimates of λ′ for A. triseriatus were less than 1 indicating that populations were declining.
Survival of A. triseriatus was similar in all treatments except interspecific controls which were substantially higher than other treatment groups. These results differ from those of studies showing that in the absence of predators, interspecific competition with A. albopictus has a greater impact than intraspecific competition on A. triseriatus [47]–[51] but support a meta-analysis suggesting competitive equivalence [59]. The cause of this difference from other studies is unknown, but release from competition may have occurred due to the addition of supplemental larval food during the experiment. Also, differences in the timing of development and emergence between the species may have been contributing factors. Specifically, male and female A. albopictus emerged as adults within ∼8–10 days whereas A. triseriatus required ∼11–15 days. Therefore, A. triseriatus in interspecific treatments experienced release from competition after A. albopictus developed into pupae (a non-feeding stage) which would have occurred before the addition of more larval food resources 11 and 14 days after larvae were added to containers. Additionally, cohorts were synchronized and so there were few or no stragglers as larvae, another sign of release from competition.
Capture and consumption of prey by predators may decrease development time of prey by releasing survivors from competition [44], [59], [62]–[63] or through selective consumption of slow developing prey [64]. Shorter development times were observed for A. albopictus in the predator present and prey removal treatments than in the controls. Also, survivorship to adulthood was approximately four times higher in controls than in the former two treatments. Together these effects indicate that competition was occurring because thinning of mosquito prey by C. appendiculata or prey removal accelerated development. Similar developmental times to metamorphosis of A. albopictus between the predator and removal treatments suggest that C. appendiculata did not reduce activity and foraging of prey [65], prolonging development. These results are consistent with Griswold and Lounibos [44] showing decreased development time of A. albopictus, but not A. triseriatus, in the presence of C. appendiculata. Corethrella appendiculata is a size-limited predator and so the lack of predator effects on A. albopictus males may be in part due to rapid development and the achievement of larger sizes less vulnerable to C. appendiculata.
Prey exposed to predation may experience enhanced growth among survivors [44], [59], [62]–[63] from competitive release or size-selection favoring larger individuals among survivors [64], [66]. This effect is especially pronounced among size-limited predators where prey may reach a size refuge [24]. We observed enhanced growth of A. albopictus in removal treatments simulating daily thinning of prey by C. appendiculata but not in the predator present treatments. The lack of enhanced growth of A. albopictus in the predator present treatments suggests that nonconsumptive effects of C. appendiculata oppose thinning effects of predation for mosquito growth. Lounibos et al. [67] observed smaller sized A. triseriatus adult females from tires with T. rutilus compared to tires where T. rutilus was absent and suggested a plausible mechanism; reduced foraging activity leading to decreased size at metamorphosis. Studies have since established that A. triseriatus, and to a lesser extent A. albopictus, do reduce activity in the presence of T. rutilus and C. appendiculata [33]–[34], [65], [35]. In the current study, if A. albopictus reduced activity and foraging in presence of C. appendiculata we would expect associated lengthening of development time. However, we observed the exact opposite result. The size and timing of metamorphosis for organisms with complex life cycles is tightly associated with variation in environment. Field studies on spatial and temporal patterns of phenotypic variation in size and timing of metamorphosis of the mayfly Baetis bicaudatus showed that sizes were smallest in streams with predatory fish and the sizes decreased with predatory stonefly density [68]. Predator-mediated reductions in sizes of mayflies were associated with accelerated development. These effects were independent of resources, competitor densities, and other physical and chemical variables. The authors proposed that variation in size and timing of metamorphosis represented adaptive developmental plasticity. Although definitive evidence is lacking, the results of this study are consistent with those of other studies showing adaptive developmental plasticity in response to variation in risk of predation for A. albopictus.
Predator intimidation in the absence of prey culling may have strong influence over phenotypic traits of prey [10], [17] including alterations in lifespan [35]. Thinning of prey by removal and the presence of C. appendiculata produced opposite effects on survivor function estimates of adults of A. albopictus from intraspecific treatments. In the absence of C. appendiculata, thinning of prey increased survival probability (longest lifespans) through competitive release whereas thinning of prey in the presence of C. appendiculata reduced survival probability (shortest lifespans). Thus, the impact of intimidation by C. appendiculata comes at an energetic cost to prey realized by reductions in growth and survival probability of adults. This is one of a few studies that have identified increased probability of death (life-shortening) effects on mosquitoes due to trait mediated effects of predators [35]. However, our experiment did not independently assess trait and density mediated effects of C. appendiculata. That is, no treatments included only predator cues or predator cues in combination with simulated daily reductions in density via prey removal. Rather, our treatments were limited to lethality, lethality + intimidation, and controls.
Biological control agents targeting the immature stages of mosquitoes (e.g., predatory fish, crustaceans and insects; parasitic fungi and nematodes) are assumed to reduce risk of disease transmission through reductions in the density of adult vector mosquitoes. Observed predator effects on A. albopictus suggest an unanticipated benefit of biological control by predators i.e., the impact of intimidation resulting in reductions in growth, and associated fecundity, and daily survival probability of adults. These effects have yet to be observed under field conditions and so should be interpreted with caution. Vector-borne diseases are sensitive to altered vector lifespan, especially when the extrinsic incubation period of the pathogen approaches life expectancy. Control interventions that reduce the daily survival probability of adult mosquitoes via insecticides or the life-shortening bacteria Wolbachia are predicted to reduce transmission. In the current experiment, predator effects on A. albopictus survival probability of adults were complex as they also depended on interspecific interactions with A. triseriatus. Specifically, the life-shortening effects of C. appendiculata were not observed in A. albopictus adults reared with A. triseriatus during the immature stages. Along the same lines, adult life-shortening effects attributable to high larval densities during the immature stages (controls) were observed for A. albopictus in the intra but not interspecific treatments with A. triseriatus. Interspecific interactions with A. triseriatus, but not conspecifics, confers a lifespan advantage for A. albopictus.
The results of this study highlight the need to improve our understanding of how mosquitoes respond to predators that are naturally present or intentionally released in the environment as a means of controlling mosquito pests and vectors of disease agents. Different pathways contribute to effects of predators on phenotypic traits and population growth of mosquitoes [12]. The current study was a necessary starting point to first establish whether the thinning effect of simulated predation were commensurate with consumptive and nonconsumptive effects of C. appendiculata. In some instances, there were nonconsumptive (trait mediated) effects of predation. In this context, studies should aim to make use of treatment manipulations that enable further dissection of density reduction, predation cues, and selective predation on phenotypic traits of mosquito prey and population growth.
Acknowledgments
The authors thank L.P. Lounibos and E. Blosser for kindly provided us with Aedes triseriatus eggs and Corethrella appendiculata larvae and use of an electrobalance to measure weights of mosquitoes. We thank L.P. Lounibos and J.R. Rey for reviewing earlier versions of the manuscript and helpful communications with S.A. Juliano.
Funding Statement
This study was supported by funds from the Institute of Food and Agricultural Sciences, University of Florida. Publication of this article was funded in part by the University of Florida Open-Access Publishing Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sih A, Crowley P, McPeek M, Petranka J, Strohmeier K (1985) Predation, competition, and prey communities: A review of field experiments. Ann Rev Ecol Syst 16: 269–311. [Google Scholar]
- 2. Abrams PA (2000) The evolution of predator-prey interactions: Theory and evidence. Annu Rev Ecol Syst 31: 79–105. [Google Scholar]
- 3. Benard MF (2004) Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Evol Syst 35: 651–673. [Google Scholar]
- 4. Juliano SA (2009) Species interactions among larval mosquitoes: Context dependence across habitat gradients. Ann Rev Entomol 54: 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Relyea RA (2003) Predator cues and pesticides: A double dose of danger for amphibians. Ecol Appl 13: 1515–1521. [Google Scholar]
- 6. Relyea RA, Hoverman JT (2008) Interactive effects of predators and a pesticide on aquatic communities. Oikos 117: 1647–1658. [Google Scholar]
- 7. Relyea RA (2012) New effects of Roundup on amphibians: Predators reduce herbicide mortality; herbicides induce antipredator morphology. Ecol Appl 22: 634–647. [DOI] [PubMed] [Google Scholar]
- 8. Grabowski JH (2004) Habitat complexity disrupts predator-prey interactions but not the trophic cascade on oyster reefs. Ecology 85: 995–1004. [Google Scholar]
- 9. Johnson DW (2006) Predation, habitat complexity, and variation in density-dependent mortality of temperate reef fishes. Ecology 87: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 10. Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84: 1083–1100. [Google Scholar]
- 11. Bolnick DI, Preisser EL (2005) Resource competition modifies the strength of trait-mediated predator-prey interactions: A meta-analysis. Ecology 86: 2771–2779. [Google Scholar]
- 12.Priesser EL, Bolnick DI (2008) The many faces of fear: comparing the pathways and impacts of noncumsumptive predator effects on prey populations. PLoS ONE 3: [DOI] [PMC free article] [PubMed]
- 13. Fill A, Long EY, Finke DL (2012) Non-consumptive effects of a natural enemy on a non-prey herbivore population. Ecol Entomol 37: 43–50. [Google Scholar]
- 14. Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68: 619–640. [Google Scholar]
- 15. Lima S (1998) Non-lethal effects in the ecology of predator-prey interactions. BioScience 48: 25–34. [Google Scholar]
- 16. Barry M (1994) The costs of crest induction for Daphnia carinata . Oecologia 97: 278–288. [DOI] [PubMed] [Google Scholar]
- 17. Priesser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86: 501–509. [Google Scholar]
- 18. Hoverman JT, Relyea RA (2009) Survival trade-offs associated with inducible defences in snails: the roles of multiple predators and developmental plasticity. Funct Ecol 23: 1179–1188. [Google Scholar]
- 19. Dahl J, Peckarsky BL (2002) Induced morphological defenses in the wild: predator effects on a mayfly, Drunella coloradensis . Ecology 83: 1620–1634. [Google Scholar]
- 20. Johansson F (2002) Reaction norms and production costs of predator-induced morphological defences in a larval dragonfly (Leucorrhinia dubia: Odonata). Can J Zool 80: 944–950. [Google Scholar]
- 21. Sih A (1986) Antipredator responses and the perception of danger by mosquito larvae. Ecology 67: 434–441. [Google Scholar]
- 22. Hetchtel LJ, Juliano SA (1997) Effects of a predator on prey metamorphosis: plastic responses by prey or selective mortality? Ecology 78: 838–851. [Google Scholar]
- 23. Day T, Abrams PA, Chase JM (2002) The role of size-specific predation in the evolution and diversification of prey life histories. Evolution 56: 877–887. [DOI] [PubMed] [Google Scholar]
- 24. Urban MC (2007) The growth-predation risk trade-off under a growing gap-limited predation threat. Ecology 88: 2587–2597. [DOI] [PubMed] [Google Scholar]
- 25. Reznick DN, Butler MJ IV, Rodd FH, Ross P (1996) Life-history evolution in guppies (Poecilia reticulate) 6. Differential mortality as a mechanism for natural selection. Evolution 50: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 26. Juliano SA, Lounibos LP (2005) Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett 8: 558–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yee DA, Allgood D, Kneitel JM, Kuehn KA (2012) Constitutive differences between natural and artificial container mosquito habitats: Vector communities, resources, microorganisms, and habitat parameters. J Med Entomol 49: 482–491. [DOI] [PubMed] [Google Scholar]
- 28. Alto BW, Lounibos LP, Mores CN, Reiskind MH (2008) Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc R Soc B 275: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bevins SN (2008) Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae). Biol Invas 10: 1109–1117. [Google Scholar]
- 30. Alto BW, Lounibos LP, Higgs S, Juliano SA (2005) Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology 86: 3279–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muturi EJ, Kim C-H, Alto BW, Schuler MA, Berenbaum MR (2011) Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Trop Med Int Health 16: 955–964. [DOI] [PubMed] [Google Scholar]
- 32. Beketov MA, Liess M (2007) Predation risk perception and food scarcity induce alterations of life-cycle traits of the mosquito Culex pipiens . Ecol Entomol 32: 405–410. [Google Scholar]
- 33. Kesavaraju B, Juliano SA (2004) Differential behavioral responses to water-borne cures to predation in two container dwelling mosquitoes. Annals Entomol Soc Am 97: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kesavaraju B, Alto BW, Juliano SA, Lounibos LP (2007) Behavioral responses of larval container mosquitoes to a size-selective predator. Ecol Entomol 32: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Costanzo KS, Muturi EJ, Alto BW (2011) Trait-mediated effects of predation across life-history stages in container mosquitoes. Ecol Entomol 36: 605–615. [Google Scholar]
- 36. Lounibos LP (2002) Invasions by insect vectors of human diseases. Annu Rev Entomol 47: 233–266. [DOI] [PubMed] [Google Scholar]
- 37. Kaplan L, Kendell D, Robertson D, Livdahl T, Khatchikian C (2010) Aedes aegypti and Aedes albopictus in Bermuda: extinction, invasion, invasion and extinction. Biol Invasions 12: 3277–3288. [Google Scholar]
- 38. Juliano SA, Lounibos LP, O'Meara GF (2004) A field test for competitive effects of Aedes albopictus on Aedes aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia 139: 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lounibos LP, O'Meara GF, Escher RL, Nishimura N, Cutwa M, et al. (2001) Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Invasions 3: 151–166. [Google Scholar]
- 40. Kesavaraju B, Damal K, Juliano SA (2008) Do natural container habitats impede invader dominance? Predator-mediated coexistence of invasive and native container-dwelling mosquitoes. Oecologia 155: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Juliano SA, Reminger L (1992) The relationship between vulnerability to predation and behavior of larval tree-hole mosquitoes: geographic and ontogenetic differences. Oikos 63: 465–467. [Google Scholar]
- 42. Kesavaraju B, Juliano SA (2008) Behavioral responses of Aedes albopictus to a predator are correlated with size-dependent risk of predation. Ann Entomol Soc Am 101: 1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Griswold MW, Lounibos LP (2005a) Does differentially predation permit invasive and native mosquito larvae to coexist in Florida? Ecol Entomol 30: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Griswold MW, Lounibos LP (2005b) Competitive outcomes of aquatic container Diptera depend on predation and resource levels. Ann Entomol Soc Am 98 673–681: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alto BW, Kesavaraju B, Juliano SA, Lounibos LP (2009) Stage-dependent predation on competitors: consequences for the outcome of a mosquito invasion. J Anim Ecol 78: 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Juliano SA, Lounibos LP, Nishimura N, Greene K (2010) Your worst enemy could be your best friend: predator contributions to invasion resistance and persistence of natives. Oecologia 162: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Livdahl TP, Willey MS (1991) Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus . Science 253: 189–191. [DOI] [PubMed] [Google Scholar]
- 48. Novak MG, Higley LG, Christianssen CA, Rowley WA (1993) Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera: Culicidae) through replacement series experiments. Environ Entomol 22: 311–318. [Google Scholar]
- 49. Teng H-J, Apperson CS (2000) Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperatures. J Med Entomol 37: 40–52. [DOI] [PubMed] [Google Scholar]
- 50. Bevins SN (2007) Timing of resource input and larval competition between invasive and native container-inhabiting mosquitoes (Diptera: Culicidae). J Vector Ecol 32: 252–262. [DOI] [PubMed] [Google Scholar]
- 51. Yee DA, Kaufman MG, Juliano SA (2007) The significance of ratios of detritus types and microorganism productivity to competitive interactions between aquatic insect detritivores. J Anim Ecol 76: 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Werner EE (1974) The fish size, prey size, handling time relation in several sunfishes and some implications. J Fish Res Board Can 31: 1531–1536. [Google Scholar]
- 53. Relyea RA (2000) Trait-mediated indirect effects in larval anurans: reversing competition with the threat of predation. Ecology 81: 2278–2289. [Google Scholar]
- 54. Mowles SL, Rundles SD, Cotton PA (2011) Susceptibility to predation affects trait-mediated indirect interactions by reversing interspecific competition. PLoS ONE 6: e23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lounibos LP, Makhni S, Alto BW, Kesavaraju B (2008) Surplus killing by predatory larvae of Corethrella appendiculata: Prepupal timing and site-specific attack on mosquito prey. J Insect Behav 21: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nannini MA, Juliano SA (1998) Effects of the facultative predator Anopheles barberi on population performance of Aedes triseriatus . Ann Entomol Soc Am 91: 33–42. [Google Scholar]
- 57. Lounibos LP, Suarez S, Menendez Z, Nishimura N, Escher RJ, et al. (2002) Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J Vector Ecol 27: 86–95. [PubMed] [Google Scholar]
- 58. Sidak Z (1967) Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc 62: 626–633. [Google Scholar]
- 59. Juliano SA (2010) Coexistence, exclusion, or neutrality? A meta-analysis of competition between Aedes albopictus and resident mosquitoes. Isr J Ecol Evol 56: 325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tilman D (1982) Resource competition and community structure. Princeton University Press, Princeton, New Jersy, USA.
- 61. Sanders NJ, Gordon DM (2003) Resource-dependent interactions and the organization of desert ant communities. Ecology 84: 1024–1031. [Google Scholar]
- 62. Grill CP, Juliano SA (1996) Predicting species interactions based on behavior: predation and competition in container-dwelling mosquitoes. J Anim Ecol 65: 63–76. [Google Scholar]
- 63. Babbitt KJ, Tanner GW (1998) Effects of cover and predator size on survival and development of Rana utricularia tadpoles. Oecologia 114: 258–262. [DOI] [PubMed] [Google Scholar]
- 64. Craig JK, Burke BJ, Crowder LB, Rice JA (2006) Prey growth and size-dependent predation in juvenile estuarine fishes: experimental and model analyses. Ecology 87: 2366–2377. [DOI] [PubMed] [Google Scholar]
- 65. Kesavaraju B, Juliano SA (2010) Nature of predation risk cues in container systems: Mosquito responses to solid residues from predation. Ann Entomol Soc Am 103: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bence JR, Murdoch MW (1986) Prey size selection by the mosquitofish: relation to optimal diet theory. Ecology 67: 324–336. [Google Scholar]
- 67. Lounibos LP, Nishimura N, Escher RL (1993) Fitness of a treehole mosquito: influences of food type and predation. Oikos 66: 114–118. [Google Scholar]
- 68. Peckarsky BL, Taylor BW, McIntosh AR, McPeek MA, Lytle DA (2001) Variation in mayfly size at metamorphosis as a developmental response to risk of predation. Ecology 82: 740–757. [Google Scholar]