Abstract
Background
Active MMP-9 disruption of the extracellular matrix plays an important role in inflammatory disorders. In this study, we investigated the inflammatory role of MMP-9 and the extracellular matrix (ECM) breakdown product hyaluronan as a trigger for the postoperative intestinal inflammatory response of postoperative ileus.
Methods
A standardized intestinal surgical manipulation (SM) was performed on rats to produce ileus assessed by the oral non-digestible FITC-dextran transit assay. Isolated intestinal muscularis extracts were studied for mRNA expressions of IL-6, MMP-9 and CD44. Peritoneal MMP-9 activity was quantified using zymography. Peritoneal fluid and serum were quantified for hyaluronan and TIMP-1 levels by ELISA. Peritoneal macrophages were cultured and exposed to peritoneal fluid or synthetic hyaluronan for ELISA analysis of IL-6 and MIP-1α.
Results
Transit was significantly delayed after SM and extracts of the isolated jejunal and colonic muscularis demonstrated a significant induction of IL-6, MMP-9 and CD44 mRNAs compared to controls. Zymography confirmed significant MMP-9 activity in peritoneal fluid compared to controls. ELISA measurements showed a significant upregulation in hyaluronan and TIMP-1 in the peritoneal fluid and serum. Additionally, ELISA and RT-PCR measurements of peritoneal macrophages stimulated with postsurgical peritoneal fluid and synthetic hyaluronan resulted in higher expressions of IL-6 and MIP-1α in the macrophage supernatant.
Conclusions
Our results confirm that MMP-9 disruption in the ECM with hyaluronan release and muscularis CD44 receptor induction has the potential to trigger muscularis proinflammatory cascades which cause postoperative ileus and we suggest that MMP-9 inhibition may be a novel therapeutic approach to limit postoperative ileus.
Keywords: extracellular matrix, hyaluronan, postoperative ileus, CD44, TIMP-1, MMP-9
INTRODUCTION
The morbidity of postoperative intestinal atony and ileus is widely acknowledged, and its economic burden has been estimated in the US to be $1 billion annually 1. Recent studies have shown that prolonged postoperative intestinal ileus is incited by the generation of an enteric molecular inflammatory response that consists of the activation of the dense network of resident muscularis macrophages and their secretion of cytokines (IL-6, TNF-α), chemokines and smooth muscle inhibitory substances via inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). This local inflammatory milieu then further causes the increased expression of vascular adhesion molecules and a subsequent recruitment and extravasation of leukocytes into the circular muscle layer with a further release of potent leukocytic products which perpetuate intestinal atony. Together, these events succeed in delaying gastrointestinal transit, decrease local neuromuscular function and activate neurogenic inhibitory pathways that suppress motility along the entire gastrointestinal tract for sustained postoperative periods 2-9.
We know from earlier studies that anesthesia, laparotomy or bowel eventration do not produce a prolonged ileus, but that the physical manipulation of the gut wall itself is necessary to elicit this detrimental response 3,4. This would suggest that physical manipulation of the gastrointestinal tract leads to a disruption in the interstitial extracellular matrix (ECM) which maintains the cohesiveness of the numerous cellular constituents of the muscularis externa. The aim of this study was to analyze intestinal ECM breakdown via MMP-9 activity and explore the potential of its breakdown product hyaluronan as an inflammatory “trigger” which participates in the postoperative inflammatory response generated by the dense network of normally quiescent muscularis macrophages which leads to the deleterious state of postoperative ileus.
The key role of MMP-9 and TIMP-1 in the progression, prognosis and treatment of gastrointestinal cancers is being intensively investigated10,11. A significant body of evidence has also developed in the inflammatory bowel disease literature over the past decade indicating that increased mucosal MMP-9 activity during episodes of inflammation participates in the destruction of the epithelial barrier, which subsequently exposes the immunologically active lamina propria to bacterial antigens resulting in the production of proinflammatory mediators that further aggravate the pathology12,13. Interestingly, ECM fragments of hyaluronan have been shown to directly be proinflammatory in cell culture conditions on peritoneal macrophages via NF-κB activation14 through CD44 receptor binding and on endothelial cells through TLR4 ligand activity15.
To our knowledge, however, no previous study has focused on the pathophysiological role of MMP-9 activity on the ECM within the intestinal muscularis in any disease. Interestingly, in this study, we show that manipulation induced postoperative ileus is associated with a rapid and sustained induction of MMP-9 activity within the postsurgical muscularis externa which results in a dramatic increase in peritoneal hyaluronan levels bathing the gut wall. Furthermore, the receptor for hyaluronan (CD44) is postoperatively induced and postoperative peritoneal fluid and exogenous hyaluronan possess significant proinflammatory potential on cultured peritoneal macrophages. These data indicate that limiting the proinflammatory activity of MMP-9 generated hyaluronan may be a novel approach to limiting the morbidity of postoperative ileus.
METHODS
Animals
Sprague Dawley male rats (280-320 g) were purchased from Charles River Laboratories (Sulzfeld, Germany) and maintained in a pathogen-free animal facility at the University of Bonn with standard rat chow and tap water supplied ad libitum. They were allowed to acclimatize at least five days prior to experimental manipulation. The animal protocol was approved by the District Government of Köln, Germany.
Intestinal Manipulation
The entire intestine of the animals was subjected to a standardized, moderate SM as described previously 16. In brief, animals were anesthetized with continuous isoflurane inhalation (DeltaSelect, Pfullingen, Germany) and a midline abdominal incision was performed. The cecum and small bowel were eventrated and placed onto moist gauze outside of the abdominal cavity and kept moist with saline. Next, the entire small bowel and colon were manipulated using moist sterile cotton applicators in a standardized fashion. After manipulation, the intestine was replaced and the laparotomy closed by two layers of continuous sutures. Age-matched, non-manipulated, naÿe animals without surgery served as controls (N=6 each group).
In vivo Gastrointestinal Transit
Gastrointestinal transit was measured in controls and manipulated animals 24 hrs postoperatively by evaluating the gastrointestinal distribution of fluorescein-labeled dextran (molecular weight = 70000, Sigma-Aldrich, Munich, Germany) as previously described 17. For statistical analysis, a geometric center (GC) was calculated for the median distribution of fluorescein-labeled dextran along the gastrointestinal tract as previously described 17.
Peritoneal fluid and Serum
Postoperatively, after 0, 3, 6 and 24 hrs, peritoneal fluid (2 ml/animal) was obtained and preserved at -20° (n=6). In brief, sterile saline was injected intraperitoneally at the defined time points after abdominal surgery in manipulated or control animals and peritoneal fluid was recollected and analyzed for ECM components and cytokines. Venous blood samples (8 ml/animal) from the different animal groups were withdrawn at the defined time points from the inferior vena cava, centrifuged (3000 RPM for 5 min) and 2 ml of serum preserved for further measurements.
ELISA Measurement
ELISA (R&D Systems, Wiesbaden, Germany) for hyaluronan and tissue inhibitor of metalloproteinases (TIMP-1) expression levels in the peritoneal fluid and in the serum were performed according to the manufacturer’s instruction and analyzed in a Tecan Saphire microplate reader (Tecan Germany GmbH, Crailsheim, Germany). Hyaluronan was measured using an ELISA kit, which allows analysis and measurement of hyaluronan in humans and animals, and is a sandwich protein binding assay in a microplate format. The assay uses microwells coated with a highly specific hyaluronic acid binding protein (HABP) from bovine cartilage to capture hyaluronan and an enzyme-conjugated version of HABP to detect and measure hyaluronan.
Muscularis preparation for mRNA measurements
After harvesting the rat’s intestine, the entire small intestine and colon were cut into approximately 5 cm segments. Each segment was pinned in a Sylgard coated dish (Dow Corning, Wiesbaden, Germany) to remove the attached mesentery. Next, the muscularis externa was stripped off and separated from the mucosa using moist cotton applicators. The muscularis tissue samples were snap frozen and stored at -80° C. mRNA real-time RT-PCR for IL-6, CD-44 and MMP-9 expression levels in the intestinal muscularis (small bowel and colon) was performed at 3, 6 and 24 h after SM (N=6 each group).
mRNA Expression in the Intestinal Muscularis
mRNA expressions were quantified using the RNA II Extraction kit (Macherey-Nagel, Düren, Germany) followed with rDNAse treatment for DNA digest (Macherey-Nagel, Düren, Germany). cDNA synthesis was performed with the high capacity cDNA Reverse Transcription kit (Applied Biosystems, Darmstadt, Germany) in the UNO thermal block (ThermoScientific, Karlsruhe, Germany). PCR was performed using a Universal PCR Master Mix, No AmpErase (Applied Biosystems, Darmstadt, Germany) and measured and analyzed with a TaqMan (40 cycles). Sequences of the used TaqMan assays (IL-6, CD44 and MMP-9, Applied Biosystems, Darmstadt, Germany) are listed in table 1.
Table 1.
Taqman assays of nucleotide sequences of the rat (Applied Biosystems, Germany)
| Gene symbol | Assay ID | Reference Sequence | Amplicon Length |
|---|---|---|---|
| 18s-rRNA | HS99999901_s1 | - | 187 |
| IL-6 | Rn00561420_m1 | NM_012589.1 | 128 |
| CD44 | Rn00681157_m1 | NM_012924.2 | 71 |
| MMP-9 | Rn00579162_m1 | NM_031055.1 | 72 |
| MIP-1α | Rn00564660_m1 | NM_013025.2 | 76 |
IL, interleukin; MMP, matrix-metalloproteinase; MIP, macrophages inflammatory protein
Zymography
Peritoneal fluid and serum samples were analyzed for MMP-2 and MMP-9 activity. Each sample was prepared by dilution into zymogram sample buffer (Biorad, Munich, Germany) and loaded into the wells of a precast gel containing 0.1% gelatin (Invitrogen, Karlsruhe, Germany). Electrophoresis was carried out at 125V constant current for 1.5 to 2 h, until the bromphenol blue dye of the sample buffer reached the bottom of the gel. The gel was removed and incubated for 1 h at room temperature in development buffer (Biorad, Munich, Germany) in a rotary shaker. Next, after decantation, the development buffer was replaced with enzyme buffer (Biorad, Munich, Germany) and incubated at 37° C for 18 h. Staining with Coomassie Blue-250 (Biorad, Munich, Germany) and destaining were carried out at room temperature on a rotary shaker. Areas of digestion were visualized as non-staining regions of the gel. Values were expressed in pixels per background minus digested area.
Cell culture
Rats were intraperitoneally injected with 8 ml of 3% thioglycollate medium (Brewer thioglycollate Medium, Sigma-Aldrich, Munich, Germany). After 48 h, peritoneal lavage was performed and macrophages in the peritoneal fluid were harvested. In brief, rats were injected 50 ml of harvest medium and lightly massaged. After 3 min, about 30-40 ml of medium was extracted from the intraperitoneal cavity and put in two Falcon tubes (Labomedic, Bonn, Germany). This procedure was performed twice. The Falcon tubes were centrifuged and peritoneal macrophages were isolated. After isolating, cells were counted using a Neubauer haemocytomer (Assistant, Sondheim, Germany), plated in culture flasks with DMEM Buffer (Lonza, Cologne, Germany) and fetal calf serum (FCS: Lonza, Cologne, Germany) and placed in a CO2 incubator (3 mil cells/well/6-well-plate) for 72 hours. Prior to experiments, cell culture medium was exchanged with FCS free medium and after 12 hours exposed to peritoneal fluid. After 1 hour of incubation with peritoneal fluid, the supernatant fluid was removed and substituted with fresh culture medium. After an additional 6 hours of incubation the new supernatant fluid was taken, stored and analyzed by ELISA for IL-6 (R&D Systems, Wiesbaden, Germany). FCS free macrophages were also exposed to synthetic low molecular weight hyaluronan (<250 kDa) (50μg/ml) dissolved in DMEM buffer for 6 and 24 hours. IL-6 and MIP-1α mRNAs were measured in extracts of the incubated peritoneal macrophages that were collected from the wells, centrifuged, stored and extracted for PCR Taqman assays (Applied Biosystems, Darmstadt, Germany), see table 1 for sequences.
Statistical Analysis Data analysis
The results are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using an unpaired Student’s t-test for single comparisons or ANOVA for multiple comparisons using the Bonferonni post hoc test. A probability level of p ≤ 0.05 was considered statistically significant.
RESULTS
In Vivo Gastrointestinal Transit
We established that our animals developed a clinically relevant postoperative pan-enteric dysmotility by measuring in vivo 90 minute gastrointestinal transit after bolus oral FITC-dextran administration in naive, non-manipulated controls and manipulated animals 24 hours after surgery. Twenty-four hours after surgical manipulation, the transit distribution histograms demonstrated a significant delay in propulsive gastrointestinal motility relative to controls. In naive control animals, the fluorescent marker reached the terminal ileum reflected by a calculated geometric center (GC) of 9.18 ± 0.67, while after SM a significant amount of dextran remained in the stomach with the marker being distributed mainly throughout the mid-small bowel (GC = 6.92 ± 1.47, p ≤ 0.003, Figure 1).
Figure 1.
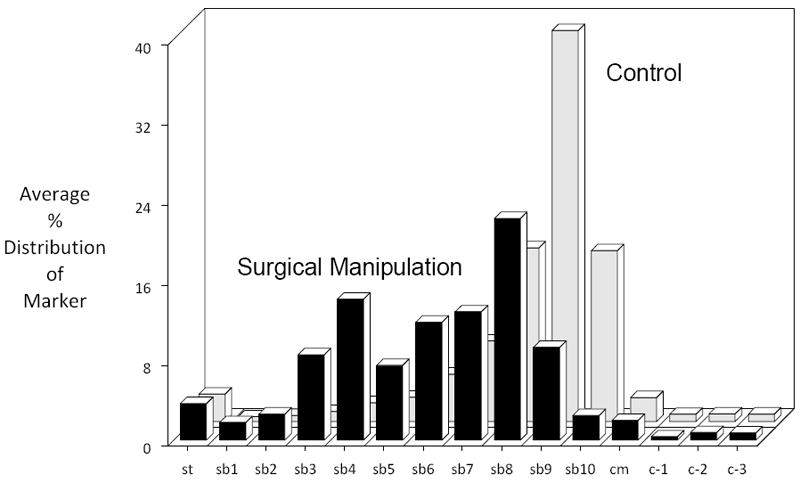
Surgical manipulation of the rat gastrointestinal produced a postoperative delay in gastrointestinal transit as measured by the lumenal distribution of an orally fed bolus of a non-digestible, non-absorbable FITC-dextran (70 kD) after 90 minutes. The calculated geometric center (GC) from the individual distribution histograms averaged 9.18 ± 0.67 for control, while after surgical manipulation the fluorescent marker was mainly distributed within the mid-jejunum and had an average GC of 6.92 ± 1.47 (N=6, p ≤ 0.003, one-way ANOVA with posthoc Bonferoni analysis).
ECM Activity in the Peritoneal Fluid and Serum
We hypothesized that surgical manipulation of the gut wall and the ensuing events results in a disruption in the extracellular matrix. Therefore, we measured the expression of matrix metalloproteinase-9 (MMP-9) mRNA and the release of MMP-9, tissue inhibitor of metalloproteinase-1 (TIMP-1) and hyaluronan into the peritoneal fluid and serum at 3, 6 and 24 hours after surgical manipulation of the rat gastrointestinal tract. PCR analysis detected a brisk rise in the expression of MMP-9 mRNA within muscularis extracts of the manipulated small and large intestines at all time points investigated (small intestine: control=1.3±0.4 vs. IM-3 hours=8.45±2.29, IM-6 hours=30.0±10.7 and IM-24 hours=37.4±6.5 relative fold increase over control) (large intestine: control=1.1±0.1 vs. IM-3 hours=23.0±10.1, IM-6 hours=30.1±9.5 and IM-24 hours=91.4±5.4 relative fold increase over control) (Figure 2A). Correlating with the increase in MMP-9 mRNA, electrophoretic zymography demonstrated a rapid and sustained increase in peritoneal fluid MMP-9 activity after SM. Densitometry quantification of the digested gelatin showed a 2.96-, 3.33- and 1.37-fold increase in enzymatic activity at 3, 6 and 24 hours after SM, respectively. A representative zymography gel is shown in Figure 2B. Associated with a persistent rise in MMP-9 was a parallel progressive increase in the release of TIMP-1 into both the peritoneum and the circulation at each of the three time points assessed (Figure 3). Although already significant at 3 hours, the largest fold increase in TIMP-1 was observed 24 hours after manipulation compared to control peritoneal fluid and serum levels (12.76- fold and 6.96-fold, respectively).
Figure 2.
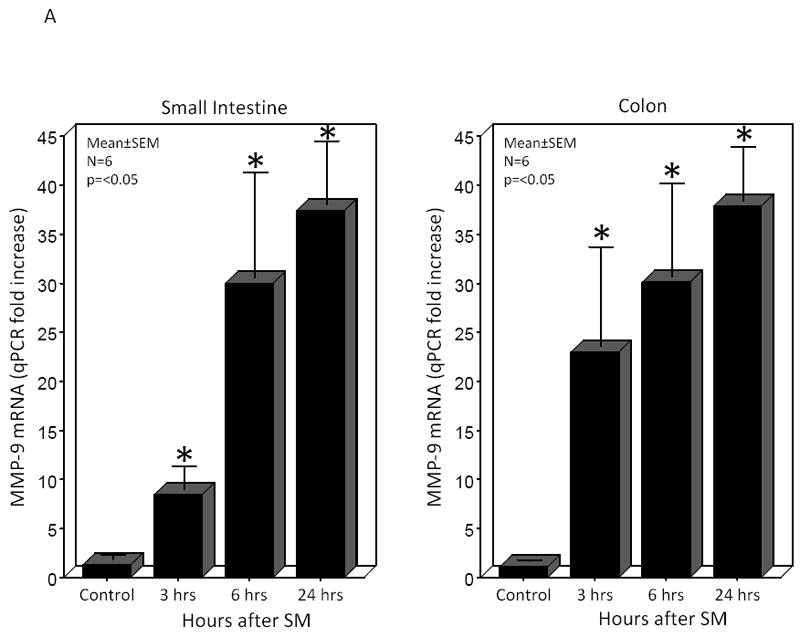
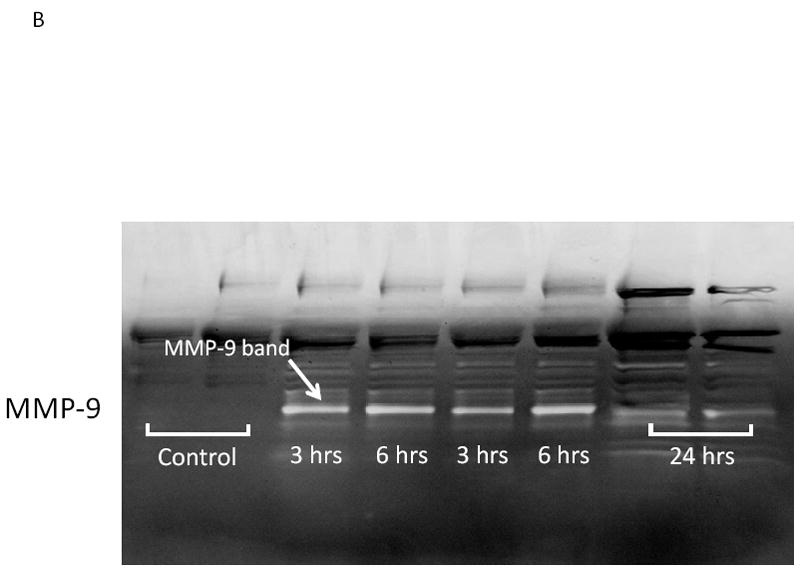
Panel 2A shows that surgical manipulation of the rat gastrointestinal tract initiated a temporal progressive increase in the qPCR expression of MMP-9 mRNA within the small intestinal (left panel) and colonic (right panel) isolated muscularis externa. MMP-9 mRNA was significantly increased at all time points measured between 3 and 24 hours after surgical manipulation compared to control (N=6, p ≤ 0.05, one-way ANOVA with posthoc Bonferoni analysis). Panel 2B is a representative electrophoretic zymography gel experiment in which a significant increase in MMP-9 enzymatic activity could be demonstrated in the peritoneal fluid harvested 3, 6 and 24 hours after surgical manipulation.
Figure 3.
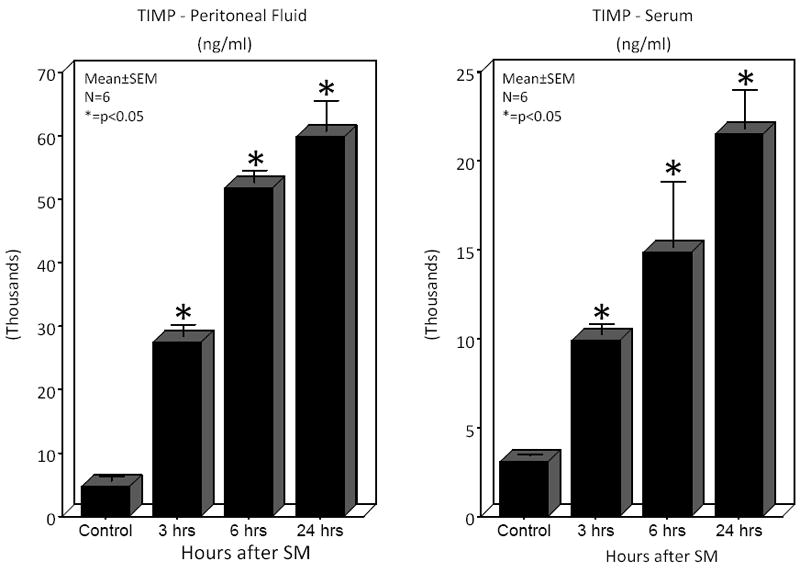
Microplate ELISA measurement of the tissue inhibitor of metalloproteinase-1 (TIMP-1) protein levels was performed on peritoneal fluid and serum harvested from controls and surgically manipulated rats 3, 6 and 24 hours after resuscitation. TIMP-1 protein levels were significantly increased rapidly within 3 hours and progressively increase through 24 hours in both the peritoneal fluid and serum harvested postoperatively (N=6, p ≤ 0.05, one-way ANOVA with posthoc Bonferoni analysis).
As a consequence of the increase in enzymatic activity on the extracellular matrix, further experiments revealed the rapid and significant release of hyaluronan into the peritoneal cavity and circulation after SM (Figure 4). In the peritoneal fluid the level of hyaluronan increased 3.01-fold 3 hours after SM, but at 6 hours after SM a massive 1139-fold release was detected with a sustained 3.77-fold increase at 24 hours. Serum hyaluronan levels were significantly lower compared to the local peritoneal fluid, but like in the peritoneal fluid a significant increased release of hyaluronan was also measured in serum with a peak 508-fold increase after 6 hours.
Figure 4.
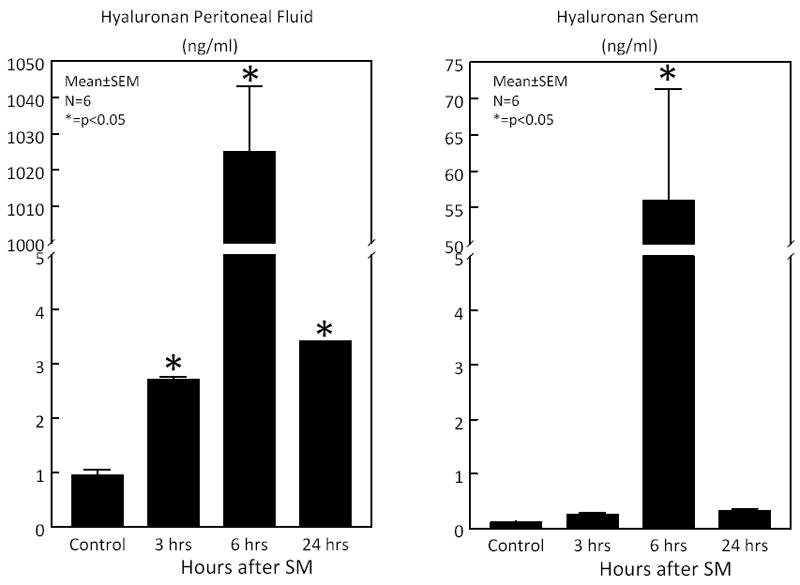
Microplate ELISA measurement of hyaluronan levels was performed on peritoneal fluid and serum harvested from controls and surgically manipulated rats 3, 6 and 24 hours after resuscitation. Hyaluronan levels were significantly increased rapidly within 3 hours, peaked at 6 hours and wane but remained elevated through 24 hours in the peritoneal fluid. Serum hyaluronan levels were significantly increased 6 hours postoperatively (N=6, p ≤ 0.05, one-way ANOVA with posthoc Bonferoni analysis).
We next sought to determine the expression level of CD44 mRNA within the muscularis, as CD44 is the membrane receptor for hyaluronan. PCR analysis of intestinal muscularis extracts demonstrated that the receptor mRNA was promptly and persistently upregulated over the 24 hours of assessment with the maximal induction occurring at the 6 hour time point (11.3-fold) for the small intestine and at 3 hours for the colon (3.4 -fold) (Figure 5).
Figure 5.
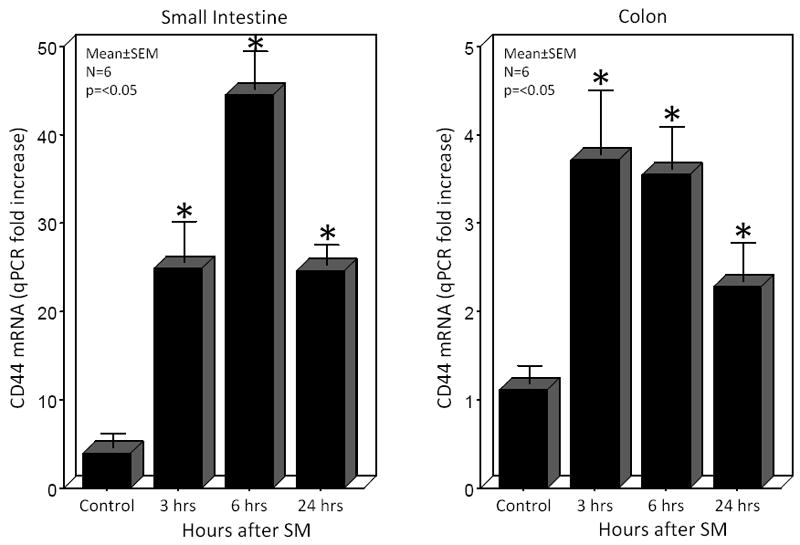
CD44, the classical hyaluronan receptor, was transcriptionally induced within the muscularis of the small and large intestines by surgical manipulation of the gastrointestinal tract. CD44 mRNA levels were significantly increased rapidly within 3 hours, peaked at 6 hours and wane but remained elevated through 24 hours in the small intestinal muscularis externa. Colonic muscularis CD44 mRNA was also postoperatively rapidly induced at 3 and gradually declined, but remained elevated through 24 hours postoperatively (N=6, p ≤ 0.05, one-way ANOVA with posthoc Bonferoni analysis).
Inflammatory Potential of Hyaluronan
Finally, we sought to explore the inflammatory potential of the hyaluronan-rich peritoneal fluid. IL-6 is a prototypical proinflammatory mediator which in this study was significantly induced within muscularis extracts of the surgically manipulated small intestine and colon at all three time points investigated, with maximal induction of IL-6 mRNA occurring after 6 hours (220.4- and 125.8-fold, respectively). In a series of cell culture experiments, isolated peritoneal macrophages were pre-incubated in culture for 1 hour with peritoneal fluid collected from controls or animals 6 hours after SM. The 6 hour harvest time point of the peritoneal fluid after surgery was chosen because the above experiments demonstrated it to be the point of maximum ECM fragment release. Incubation of freshly isolated peritoneal macrophages for 6 hours exposed to a sham procedure of media exchange lead to a measurable basal secretion of IL-6 protein (27.4±1.52 pg/ml) into the media. On the other hand, 6 hour harvested SM peritoneal fluid caused a significantly higher release of IL-6 protein (201.7±0.73 pg/ml) into the media compared to control peritoneal fluid pre-incubation macrophages. These data indicated that the hyaluronan-rich postoperative peritoneal fluid did possess inflammatory properties.
In a further series of experiments, cultured peritoneal macrophages were stimulated directly with a synthetic low molecular weight hyaluronan to determine the sole inflammatory potential of hyaluronan on macrophages. RT-PCR demonstrated a prolonged 25.6- and 24.4-fold increase in IL-6 mRNA after exposure of the cultured macrophages to hyaluronan for 6 and 24 hours, respectively. Additionally, hyaluronan caused a 2.7- and 2.24-fold increase in MIP-1α, a factor produced by macrophages that causes local inflammatory responses and induces superoxide production by neutrophils at 6 and 24 hours, respectively (Figure 6). Together, these data indicate that ECM fragments of hyaluronan released from the surgically manipulated intestine participate in generating the complex inflammatory milieu which exists within the immunologically active postoperative muscularis externa.
Figure 6.
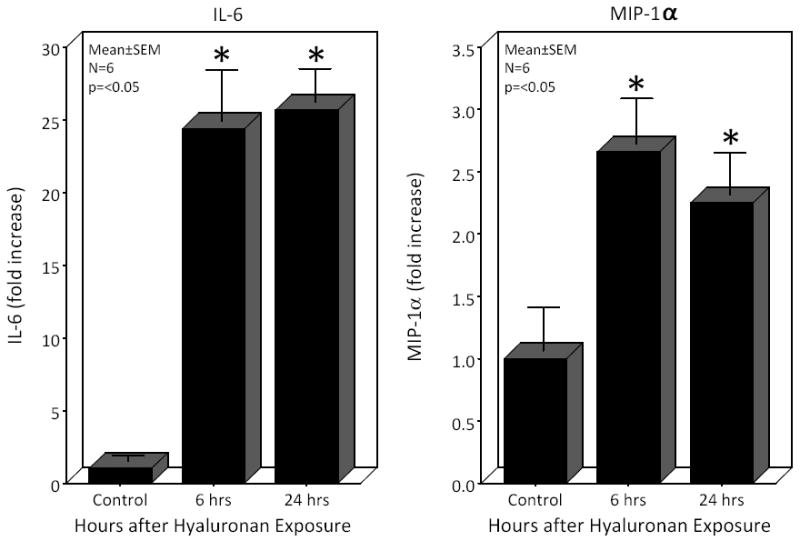
Real time PCR analysis of IL-6 and MIP-1α mRNAs from peritoneal macrophage extracts after exposure to hyaluronan (50μg/ml) for 6 or 24 hours. Hyaluronan caused the significant sustained induction of both IL-6 and MIP-1α in cultured peritoneal macrophages compared to control (N=6, p ≤ 0.05, one-way ANOVA with posthoc Bonferoni analysis).
DISCUSSION
Postoperative intestinal ileus is a common and almost obligatory feature of general visceral surgery 18. Unfortunately, postoperative ileus results in significant patient morbidity, increased duration of hospital stays and substantial hospitalization costs 19. It has been established that an intestinal inflammatory response to surgical manipulation of the gastrointestinal tract activates proinflammatory pathways, which initiate a cascade of events characterized by impaired gastrointestinal transit, decreased muscular and neuromuscular function, and increased neurogenic inhibitory activity along the entire gastrointestinal tract 5,6,20,21. In this study, we investigated the role of matrix metalloproteinases and the muscularis extracellular matrix as a significant triggering component, which could initiate and maintain the postoperative local molecular and cellular inflammatory responses following intestinal manipulation that produce postoperative ileus.
Matrix metalloproteinases (MMPs) constitute a family of enzymes capable of degrading various extracellular matrix and basement membrane components playing a role in matrix turnover. They also activate and degrade signaling molecules, such as cytokines and chemokines. MMPs are involved in inflammation and have been implicated in tissue degradation 22,23. As in other organs, MMPs appear to play an important role in intestinal pro-inflammatory signaling cascades, as has been previously demonstrated for Crohn’s disease 24. Additionally, CD44 and active proteolytic MMP-9 are found associated on migrating cells and can mediate collagen IV degradation and promote cell invasion.
In support of the hypothesized important role of the extracellular matrix in postoperative ileus, our data demonstrate that MMP-9 mRNA is significantly induced within the muscularis of the small bowel and colon in a time-dependent manner following intestinal manipulation. Additionally, we measured a rapid and sustained dramatic increase of the active form of MMP-9 protein in peritoneal fluid after surgical manipulation of the bowel wall. Part of the rapid increase in activated MMP-9 protein could be due to the release of hyaluronan during gut manipulation itself, since hyaluronan is known to convert the inactive forms of MMP-2 and MMP-9 to their active forms 25. Hence, MMP activation could occur through mechanical disruption of the matrix, increased activation and upregulated gene transcription.
Parallel to the postsurgical increased induction and activity of MMP-9, we investigated the endogenous tissue inhibitor of MMPs - tissue inhibitors of matrix metalloproteinases-1 (TIMP-1). TIMP-1 plays an important counter-regulatory role during various disease states 22,23. In an effort to understand the inhibitory role of TIMP-1 within the postsurgical degradation of the intestinal extracellular matrix, we measured the significant increased expression of TIMP-1 in serum and peritoneal fluid. An augmentation in TIMP-1 activity would be expected to function as an antiinflammatory response limiting the severity of postoperative ileus, as has been hypothesized in patients with severe sepsis 26.
Hyaluronan is a ubiquitous molecule and a major component of the peri/extracellular matrix which is synthesized into an extensive polymer. However, its molecular weight is variable. It is known that during tissue injury, inflammation and clearance of cellular debris, fragmented hyaluronan is released into the circulation by increased MMP activity 27. Throughout the development of injury, hyaluronan regulates cell motility, invasion, and proliferation by binding to CD44 receptors and activating intracellular signaling pathways. Serum elevations in hyaluronan have been measured in many inflammatory disorders and the fragmented extracellular matrix components (hyaluronan and fibronectin) themselves have been shown to function as proinflammatory stimuli to macrophages 28,29. Interestingly, the inflammatory properties depend on the size of the polymer. Low molecular weight hyaluronan has been shown to be pro-inflammatory and stimulatory for cell proliferation and migration, whereas high molecular weight hyaluronan is inhibitory 30.
With this understanding, our results demonstrate a 1000-fold upregulation in released hyaluronan 6 hours after SM in the serum and peritoneal fluid compared to controls. This observation strengthens our premise that low molecular weight hyaluronan, as an extracellular matrix breakdown product plays an important role in triggering the postoperative inflammatory signal cascade via the CD44 or TLR4 on resident intestinal muscularis macrophages and invading leukocytes which subsequently cause the functional impairment of the gastrointestinal tract15,31. In support of this hypothesis, we were able to provoke a significant pro-inflammatory response in cultured peritoneal macrophages stimulated with synthetic low molecular weight hyaluronan in comparison to controls. Additionally, a significant increase in IL-6 transcription along with IL-6 and MIP-1α protein secretion was observed in the cultured macrophages and their supernatant after exposure to peritoneal fluid harvested from manipulated animals compared to fluid harvested from controls. The fact that incubated peritoneal macrophages can be stimulated and activated with synthetic low molecular weight hyaluronan and additionally with peritoneal fluid of manipulated animals provides crucial information regarding the proinflammatory role of the disrupted intestinal extracellular matrix, especially as a trigger for macrophage activation and the development of postoperative ileus.
The hyaluronan receptor CD44 is a multifunctional, ubiquitously expressed glycoprotein that participates in cell adhesion and leukocyte recruitment to sites of inflammation by its ligands glycosaminoglycan and hyaluronan32. During the development of injury, hyaluronan regulates cell motility, invasion, and proliferation by binding to CD44 receptors and activating intracellular signaling pathways33. In our study, the enhanced expression of CD44 mRNA in the intestinal muscularis and the parallel local and systemic release of its ligands (fragmented hyaluronan) from the intestinal extracellular matrix of the surgically manipulated intestine strongly suggest their potential role in synergistically triggering known pro-inflammatory cascades in intestinal macrophages, which participate in causing the generation of copious amounts of gut-derived inflammatory mediators (IL-6, IL-1β, TNF-α, MCP-1, iNOS, COX-2, MMPs), mucosal barrier function breakdown (iNOS) and ileus (iNOS and COX-2) with the leakage of luminal toxic products5,7,20,21,34. Clearly, important mechanistic links of how the ECM-augmented postoperative inflammatory response actually causes ileus remain to be elucidated. But, as we have previously shown iNOS and COX-2 significantly contribute to postoperative bowel dysfunction.
In conclusion, this study demonstrates that intestinal manipulation results in the increased transcription and activity of MMP-9, an increase in the release of hyaluronan along with the transcriptional induction of its receptor CD44 and that hyaluronan has the potential to function as a trigger for macrophage activation. Together, these molecular events are hypothesized to participate in the synergistic triggering of the intestinal inflammatory response which is a key element in the intestinal response to the surgeon’s hand resulting in postoperative ileus.
Acknowledgments
The authors thank Susanne Schulz for excellent technical support and assistance. This study was funded Supported by the National Institute of Health (R01-GM58241, R01-DK068610, P50-GM-53789, and DK02488), the BONFOR program of the Bonn University Medical Faculty and a BONFOR grant (0-112.0032) to Overhaus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prasad M, Matthews JB. Deflating postoperative ileus. Gastroenterology. 1999;117:489–492. doi: 10.1053/gast.1999.0029900489. [DOI] [PubMed] [Google Scholar]
- 2.Kalff JC, Schraut WH, Billiar TR, et al. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000;118:316–327. doi: 10.1016/s0016-5085(00)70214-9. [DOI] [PubMed] [Google Scholar]
- 3.Kalff JC, Turler A, Schwarz NT, et al. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003;237:301–315. doi: 10.1097/01.SLA.0000055742.79045.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarz NT, Kalff JC, Turler A, et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz NT, Kalff JC, Turler A, et al. Selective jejunal manipulation causes postoperative panenteric inflammation and dysmotility. Gastroenterology. 2004;126:159–169. doi: 10.1053/j.gastro.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 6.Turler A, Moore BA, Pezzone MA, et al. Colonic postoperative inflammatory ileus in the rat. Ann Surg. 2002;236:56–66. doi: 10.1097/00000658-200207000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turler A, Kalff JC, Moore BA, et al. Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann Surg. 2006;244:220–229. doi: 10.1097/01.sla.0000229963.37544.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehner S, Schwarz NT, Hundsdoerfer R, et al. Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. 2005;137:436–446. doi: 10.1016/j.surg.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Wehner S, Behrendt FF, Lyutenski BN, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56:176–185. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochimica et Biophysica Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Yasui W, Oue N, Aung PP, et al. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86–94. doi: 10.1007/s10120-005-0320-0. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CJ, Hembry RM, Alexander A, et al. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. Journal of Clinical Pathology. 1994;47:113–116. doi: 10.1136/jcp.47.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg P, Vijay-Kumar M, Wang L, et al. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2009;296:G175–G184. doi: 10.1152/ajpgi.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble PW, McKee CM, Cowman M, et al. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. Journal of Experimental Medicine. 1996;183:2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor KR, Trowbridge JM, Rudisill JA, et al. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. Journal of Biological Chemistry. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 16.Moore BA, Overhaus M, Whitcomb J, et al. Brief inhalation of low-dose carbon monoxide protects rodents and swine from postoperative ileus. Crit Care Med. 2005;33:1317–1326. doi: 10.1097/01.ccm.0000166349.76514.40. [DOI] [PubMed] [Google Scholar]
- 17.Overhaus M, Moore BA, Barbato JE, et al. Biliverdin protects against polymicrobial sepsis by modulating inflammatory mediators. Am J Physiol Gastrointest Liver Physiol. 2006;290:G695–G703. doi: 10.1152/ajpgi.00152.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kehlet H. Postoperative ileus--an update on preventive techniques. Nat Clin Pract Gastroenterol Hepatol. 2008;5:552–558. doi: 10.1038/ncpgasthep1230. [DOI] [PubMed] [Google Scholar]
- 19.Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm. 2009;15:485–494. doi: 10.18553/jmcp.2009.15.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil. 2008;20(Suppl 1):81–90. doi: 10.1111/j.1365-2982.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalff JC, Schraut WH, Simmons RL, et al. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228:652–663. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina C, Radomski MW. Role of matrix metalloproteinases in intestinal inflammation. J Pharmacol Exp Ther. 2006;318:933–938. doi: 10.1124/jpet.106.103465. [DOI] [PubMed] [Google Scholar]
- 23.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Makitalo L, Sipponen T, Karkkainen P, et al. Changes in matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinases (TIMP) expression profile in Crohn’s disease after immunosuppressive treatment correlate with histological score and calprotectin values. International Journal of Colorectal Disease. 2009;24:1157–1167. doi: 10.1007/s00384-009-0756-5. [DOI] [PubMed] [Google Scholar]
- 25.Isnard N, Legeais JM, Renard G, et al. Effect of hyaluronan on MMP expression and activation. Cell Biol Int. 2001;25:735–739. doi: 10.1006/cbir.2001.0759. [DOI] [PubMed] [Google Scholar]
- 26.Lorente L, Martin MM, Labarta L, et al. Matrix metalloproteinase-9, -10, and tissue inhibitor of matrix metalloproteinases-1 blood levels as biomarkers of severity and mortality in sepsis. Crit Care. 2009;13:R158. doi: 10.1186/cc8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Ba ZF, Biondo A, et al. Liver endothelial cell dysfunction occurs early following hemorrhagic shock and persists despite crystalloid resuscitation. J Surg Res. 1996;63:241–247. doi: 10.1006/jsre.1996.0255. [DOI] [PubMed] [Google Scholar]
- 28.McKee CM, Lowenstein CJ, Horton MR, et al. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor kappaB-dependent mechanism. J Biol Chem. 1997;272:8013–8018. doi: 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- 29.Rockey DC, Chung JJ, McKee CM, et al. Stimulation of inducible nitric oxide synthase in rat liver by hyaluronan fragments. Hepatology. 1998;27:86–92. doi: 10.1002/hep.510270115. [DOI] [PubMed] [Google Scholar]
- 30.Bot PT, Hoefer IE, Piek JJ, et al. Hyaluronic acid: targeting immune modulatory components of the extracellular matrix in atherosclerosis. Curr Med Chem. 2008;15:786–791. doi: 10.2174/092986708783955554. [DOI] [PubMed] [Google Scholar]
- 31.Buchholz BM, Chanthaphavong RS, Bauer AJ. Nonhemopoietic cell TLR4 signaling is critical in causing early lipopolysaccharide-induced ileus. Journal of Immunology. 2009;183:6744–6753. doi: 10.4049/jimmunol.0901620. [DOI] [PubMed] [Google Scholar]
- 32.Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208–220. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- 33.Heldin P, Karousou E, Bernert B, et al. Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connect Tissue Res. 2008;49:215–218. doi: 10.1080/03008200802143323. [DOI] [PubMed] [Google Scholar]
- 34.Turler A, Schwarz NT, Turler E, et al. MCP-1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G145–G155. doi: 10.1152/ajpgi.00263.2001. [DOI] [PubMed] [Google Scholar]


