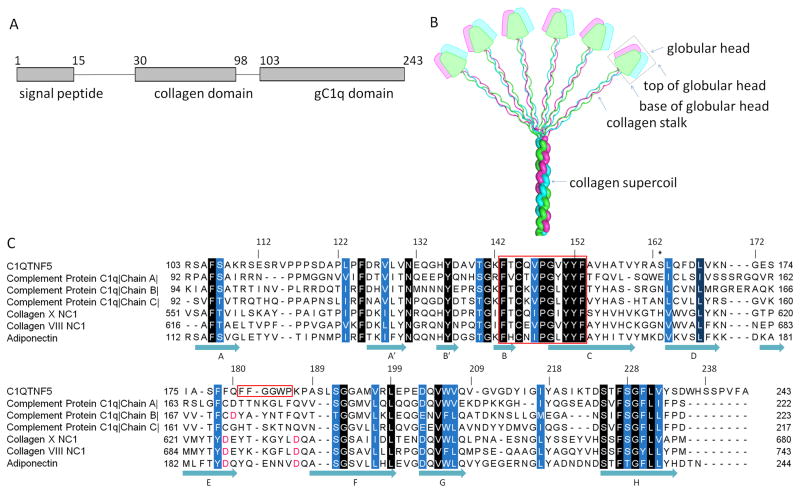
Fig. 1.
Domain organization of C1QTNF5 and sequence alignment of members of the C1q family proteins. A. The domain structure of C1QTNF5. B. A bouquet-like arrangement of the C1q family proteins. Figure adapted from (Francis et al., 2003) with permission from the authors and publisher. The three protomers are shown in magenta, green and cyan. The trimeric globular head is outlined by a black box. The top and base of the globular head are indicated by arrows. C. Sequence alignment of gC1q domains from C1q family proteins. Sequences are those of human C1QTNF5, human complement protein C1q (PDB ID: 1PK6), human collagen X NC1 (PDB ID: 1GR3), human collagen VIII NC1 (PDB ID: 1O91), and human adiponectin (PDB ID: 4DOU). Conserved residues are highlighted in white letters with background colors ranging from blue to black according to their conservation. The conserved hydrophobic motif (residues 143–153) and the F181F182G183G184W185P186 sequence are outlined in red. S163 is marked with an asterisk. Key aspartic acids coordinating with Ca2+ ions are highlighted in red. Secondary structure is assigned according to the structure of the trimeric head of human C1QTNF5.