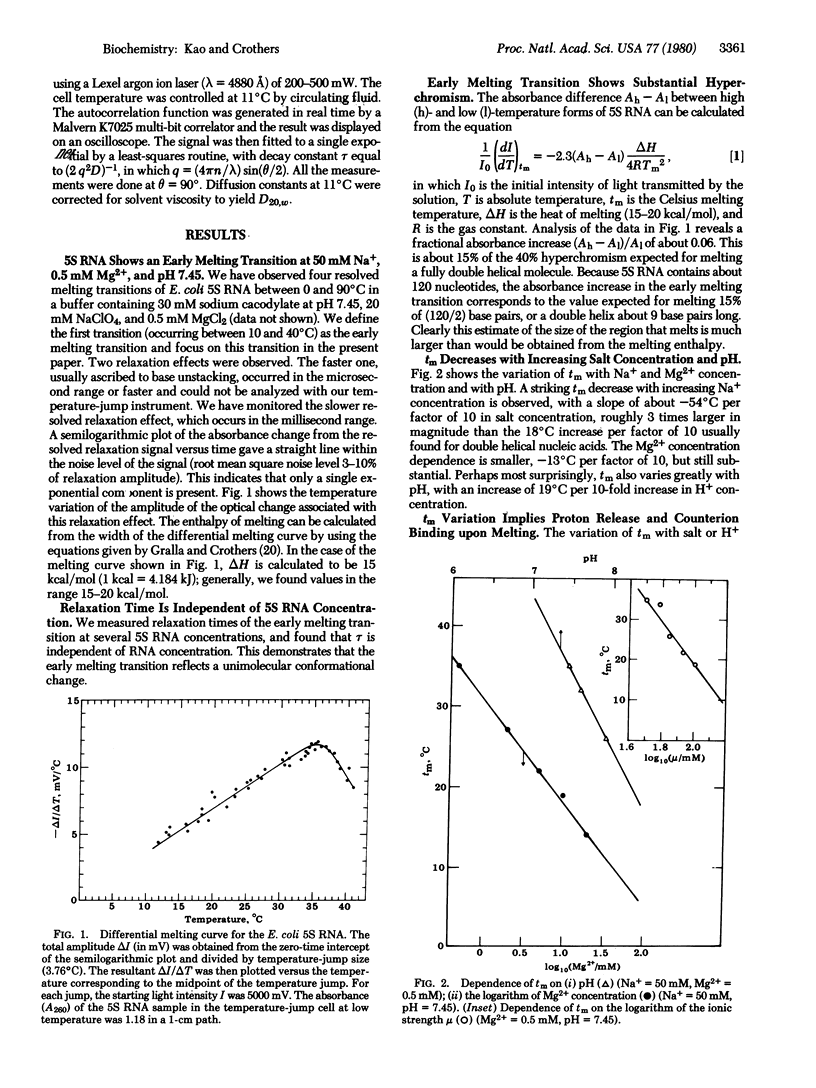
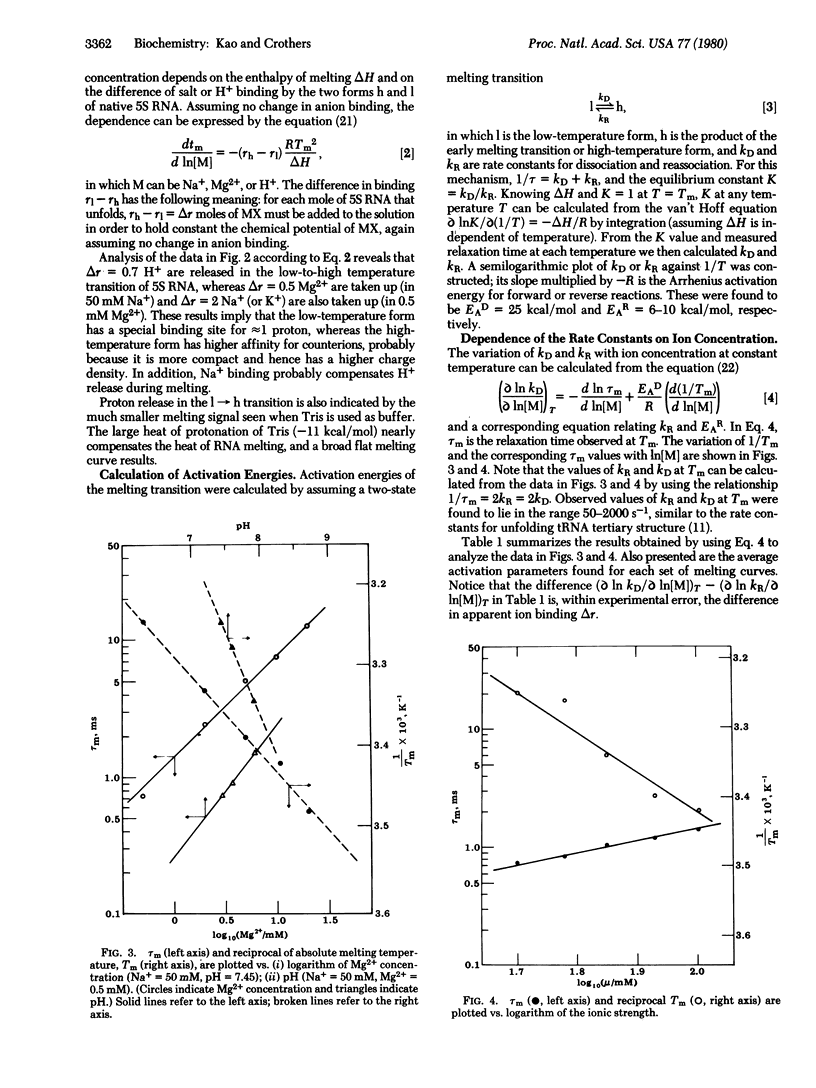
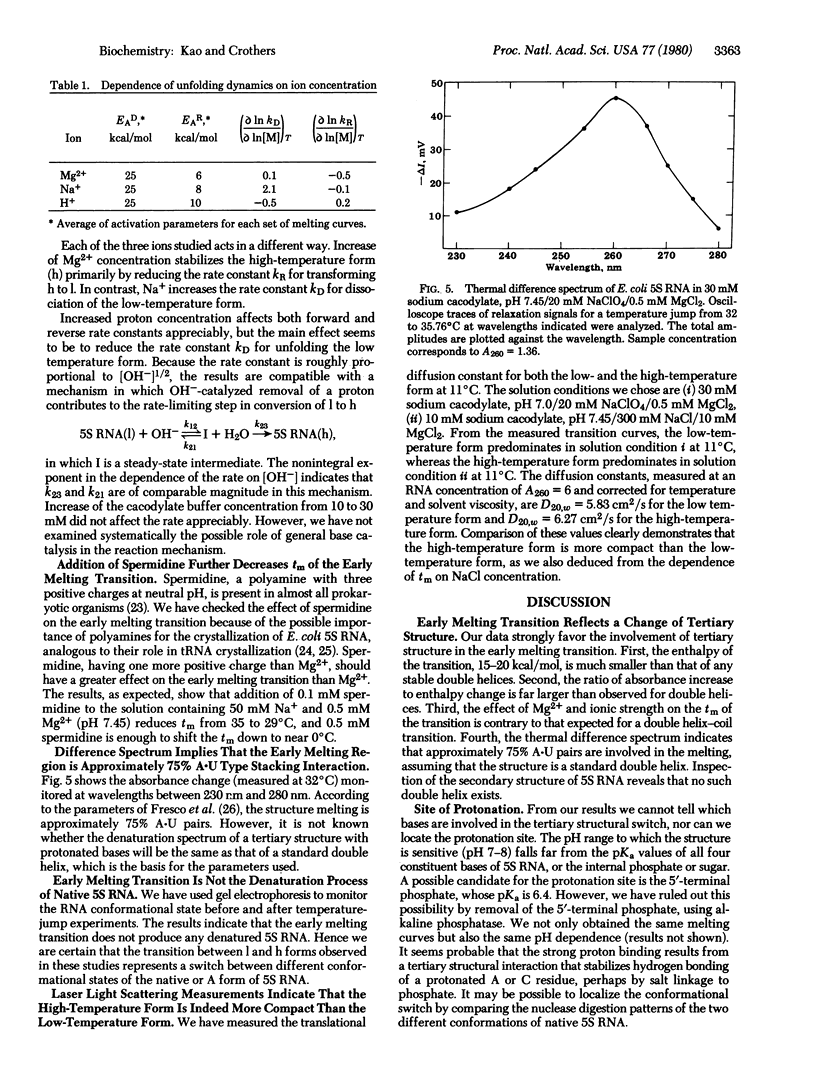
Abstract
We report temperature-jump kinetic studies of the early melting transition of Escherichia coli 5S rRNA. A single measurable relaxation time tau, independent of concentration, was found at 266 nm. We monitored the transition temperature tm for this process (in the range from 0 to 40 degrees C) as a function of Mg2+, Na+, K+, spermidine, and H+ concentrations. Contrary to the usual effect of salts on nucleic acid stability, addition of mono- and multivalent counterions decreases tm for the early melting transition. Also unexpectedly, we found a strong dependence of tm on pH in the physiological range of 7--8. Quantitative analysis of the data indicates that about 0.7 protons are release when the ordered (low-temperature) form melts, whereas about 2 NA+ (or K+) and 0.5 Mg2+ are taken up by the melted (high-temperature) form. We estimate the enthalpy of the transition to be 15--20 kcal/mol (63--84 kJ/mol) and also report the forward and reverse rate constants and activation energies for the transition, along with the influence of ions on the transition dynamics. Diffusion constant measurements reveal that the low-temperature form has a frictional coefficient about 10% larger than that of the high-temperature form. The data imply a low-temperature tertiary structure capable of binding a proton. Increase of pH, temperature, or counterion concentration (all at near-physiological values) causes a tertiary conformational switch to a more compact form that has greater counterion binding but less proton binding. We discuss possible physiological roles for the transition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. H., Wong K. P. The hydrodynamic and spectroscopic properties of 16 S RNA from Escherichia coli ribosome in reconstitution buffer. J Biol Chem. 1978 Dec 25;253(24):8759–8766. [PubMed] [Google Scholar]
- Appel B., Erdmann V. A., Stulz J., Ackerman T. Determination of base pairing in Escherichia coli and Bacillus stearothermophilus 5S RNAs by infrared spectroscopy. Nucleic Acids Res. 1979 Oct 25;7(4):1043–1057. doi: 10.1093/nar/7.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert M., Scott J. F., Reynier M., Monier R. Rearrangement of the conformation of Escherichia coli 5S RNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):292–299. doi: 10.1073/pnas.61.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P. E., Crothers D. M. Conformational changes of transfer ribonucleic acid. Relaxation kinetics of the early melting transition of methionine transfer ribonucleic acid (Escherichia coli). Biochemistry. 1972 Nov 7;11(23):4368–4374. doi: 10.1021/bi00773a025. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Cole P. E., Hilbers C. W., Shulman R. G. The molecular mechanism of thermal unfolding of Escherichia coli formylmethionine transfer RNA. J Mol Biol. 1974 Jul 25;87(1):63–88. doi: 10.1016/0022-2836(74)90560-9. [DOI] [PubMed] [Google Scholar]
- Dubin S. B. Measurement of translational and rotational diffusion coefficients by laser light scattering. Methods Enzymol. 1972;26:119–174. doi: 10.1016/s0076-6879(72)26009-8. [DOI] [PubMed] [Google Scholar]
- Ehrenberg M., Rigler R., Wintermeyer W. On the structure and conformational dynamics of yeast phenylalanine-accepting transfer ribonucleic acid in solution. Biochemistry. 1979 Oct 16;18(21):4588–4599. doi: 10.1021/bi00588a020. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A. Structure and function of 5S and 5.8 S RNA. Prog Nucleic Acid Res Mol Biol. 1976;18:45–90. [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Hilbers C. W., Robillard G. T., Shulamn R. G., Blake R. D., Webb P. K., Fresco R., Riesner D. Thermal unfolding of yeast glycine transfer RNA. Biochemistry. 1976 May 4;15(9):1874–1882. doi: 10.1021/bi00654a013. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Quigley G., Suddath F. L., Rich A. High-resolution x-ray diffraction patterns of crystalline transfer RNA that show helical regions. Proc Natl Acad Sci U S A. 1971 Apr;68(4):841–845. doi: 10.1073/pnas.68.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner J. E., Finch J. T., Klug A., Clark B. F. High-resolution x-ray diffraction studies on a pure species of transfer RNA. J Mol Biol. 1972 Dec 14;72(1):99–101. doi: 10.1016/0022-2836(72)90071-x. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Garrett R. A. Structure of 5 S ribosomal RNA from Escherichia coli: identification of kethoxal-reactive sites in the A and B conformations. J Mol Biol. 1979 Aug 25;132(4):621–636. doi: 10.1016/0022-2836(79)90378-4. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Lohman M. L., De Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976 Oct 25;107(2):145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Spierer P., Zimmermann R. A. Stoichiometry, cooperativity, and stability of interactions between 5S RNA and proteins L5, L18, and L25 from the 50S ribosomal subunit of Escherichia coli. Biochemistry. 1978 Jun 27;17(13):2474–2479. doi: 10.1021/bi00606a002. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Maglott D. M., Monro R. E. On the catalytic center of peptidyl transfer: a part of the 50 S ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:39–48. doi: 10.1101/sqb.1969.034.01.008. [DOI] [PubMed] [Google Scholar]
- Stein A., Crothers D. M. Conformational changes of transfer RNA. The role of magnesium(II). Biochemistry. 1976 Jan 13;15(1):160–168. doi: 10.1021/bi00646a025. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Vasiliev V. D., Selivanova O. M., Koteliansky V. E. Specific selfpacking of the ribosomal 16 S RNA. FEBS Lett. 1978 Nov 15;95(2):273–276. doi: 10.1016/0014-5793(78)81009-6. [DOI] [PubMed] [Google Scholar]
- Wagner R., Garrett R. A. A new RNA-RNA crosslinking reagent and its application to ribosomal 5S RNA. Nucleic Acids Res. 1978 Nov;5(11):4065–4075. doi: 10.1093/nar/5.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner H., Crothers D. M. Pathway-dependent refolding of E. coli 5S RNA. Nucleic Acids Res. 1977 Oct;4(10):3401–3414. doi: 10.1093/nar/4.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. K., Crothers D. M. Conformational changes of transfer ribonucleic acid. Comparison of the early melting transition of two tyrosine-specific transfer ribonucleic acids. Biochemistry. 1972 Nov 7;11(23):4375–4381. doi: 10.1021/bi00773a026. [DOI] [PubMed] [Google Scholar]



