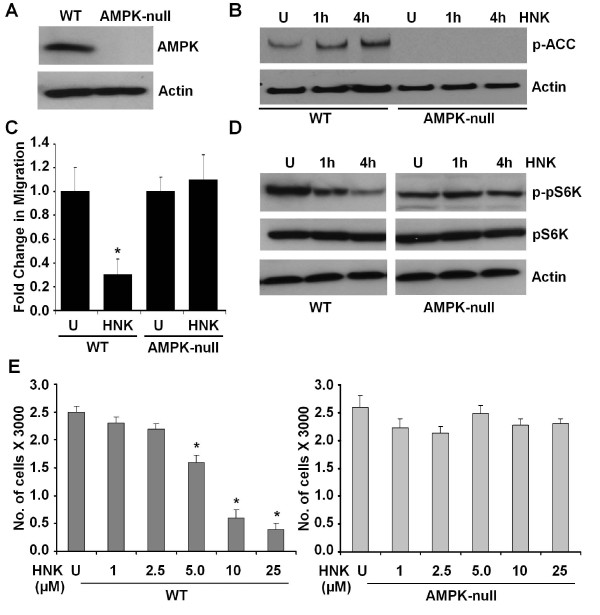
Figure 4.
AMPK knockdown abrogates honokiol-mediated increased phosphorylation of ACC, inhibition of phosphorylation of S6K, and inhibition of migration. (a) Immunoblotting for AMPK protein by using lysates from untreated MEFs derived from AMPK-WT (WT) and AMPK-knockout mice (AMPK-null). The blot was stripped and reprobed with anti-actin antibody. (b) WT and AMPK-null MEFs were treated with honokiol (HNK, 2.5 μM) for indicated time intervals. U, untreated cell. Total protein was isolated, and equal amounts of proteins were resolved with SDS-PAGE and subjected to immunoblot analysis by using specific antibodies for phosphorylated ACC (p-ACC). Anti-actin antibody was used as control. (c) WT and AMPK-null MEFs were subjected to scratch-migration assay in the presence (HNK, 2.5 μM) or absence (U) of honokiol. The plates were photographed at the identical location of the initial image (0 hours) at 24 hours. The histogram shows the fold change in migration. *P < 0.001, compared with untreated controls. All the experiments were performed thrice in triplicate. (d) WT and AMPK-null MEFs were treated with honokiol (HNK, 2.5 μM) for indicated time intervals. U, untreated cell. Total protein was isolated, and equal amounts of proteins were resolved with SDS-PAGE and subjected to immunoblot analysis by using specific antibodies for phosphorylated pS6K (p-pS6K). The membranes were reblotted by using total pS6K and actin antibody as control. (e) WT and AMPK-null MEFs were subjected to XTT assay in the presence (HNK) or absence (U) of honokiol, as indicated. The results shown are representative of three independent experiments performed in triplicate. *P < 0.001, compared with untreated controls.