Figure 2.
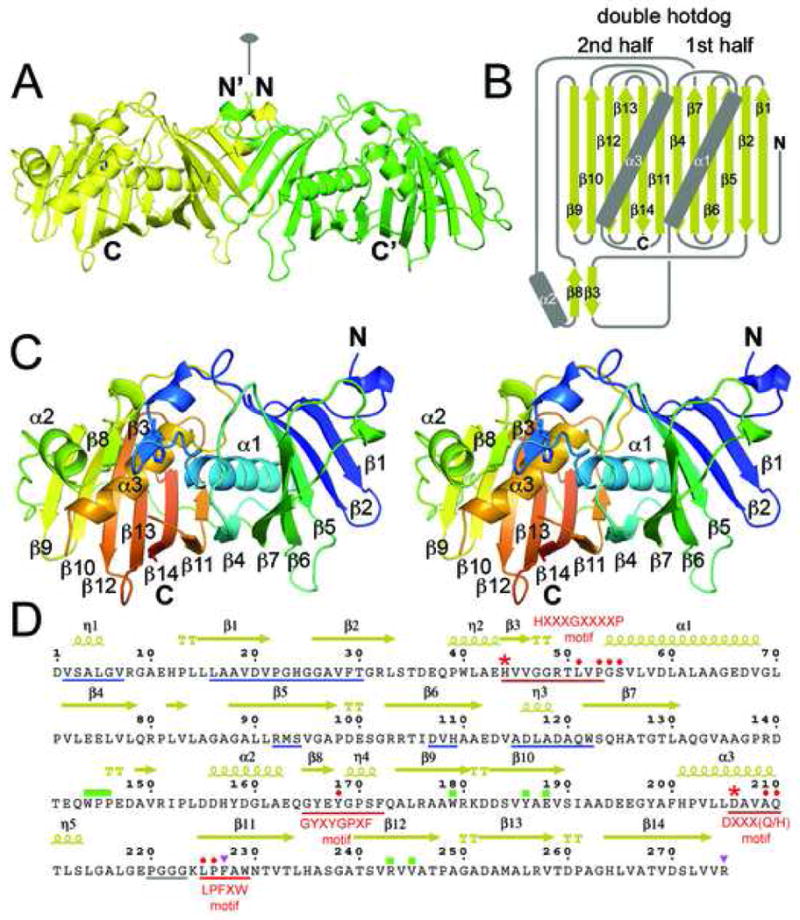
DH Structure. A) The EryDH4 dimer (one monomer is colored green for clarity) observed in the crystal lattice, showing the location the dimer interface along the twofold axis. The catalytic histidine of one monomer is represented in sticks. B) Schematic of the DH double hotdog fold (α-helices are the sausages and the β-sheet is the bun). Together, α2, β3, and β8 comprise a structural element that harbors the catalytic histidine. C) Stereodiagram of an EryDH4 monomer indicating elements of secondary structure. The protein is colored from N-terminus to C-terminus (blue to red). The catalytic histidine is represented in sticks. D) Important DH residues. Hallmark DH motifs, such as HXXXGXXXXP, are underlined in red. The catalytic histidine and aspartic acid are marked by red asterisks. Supporting active site residues are marked by red circles. Regions supporting dimerization are underlined in blue. An unobserved loop is underlined in gray. The “WPP” motif and interacting residues are marked by green rectangles. Residues hypothesized to contact ACP are marked by purple triangles. Residues indicated by “TT” help form β-turns.
