Figure 4.
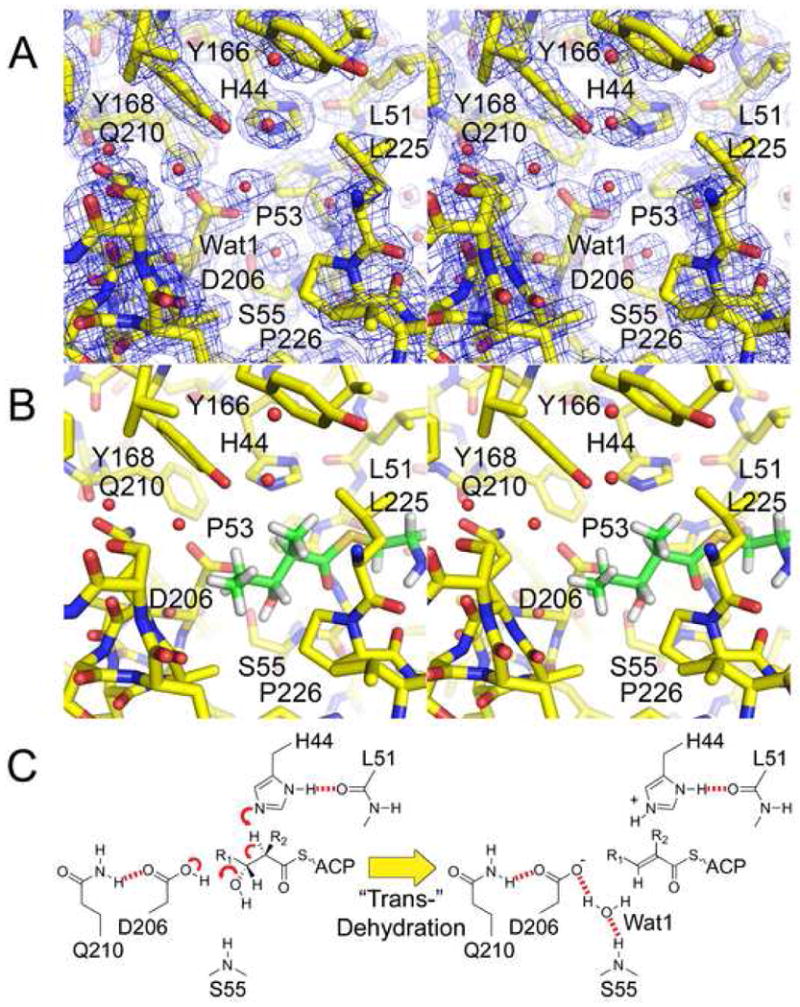
The DH Reaction. A) Stereodiagram of the 1.85 Å resolution 2Fo−Fc electron density map at the active site (contoured at 1.4 σ). The catalytic histidine, H44, and the catalytic aspartic acid, D206, are oriented by the L51 backbone carbonyl and the Q210 side chain, respectively. B) A diketide substrate mimic is modeled in the active site so that its α-hydrogen and β-hydroxyl groups are adjacent to H44 and D206, respectively. The β-hydroxyl group may partially replace Wat1 and be attracted towards the backbone NH of S55. C) Putative mechanism for the most common elimination, which yields a trans- double bond. In cis-dehydration (e.g. RifDH10) the β-hydroxyl group may remain in the same position; however, the R1 acyl chain and the β-hydrogen would be swapped.
