Figure 5.
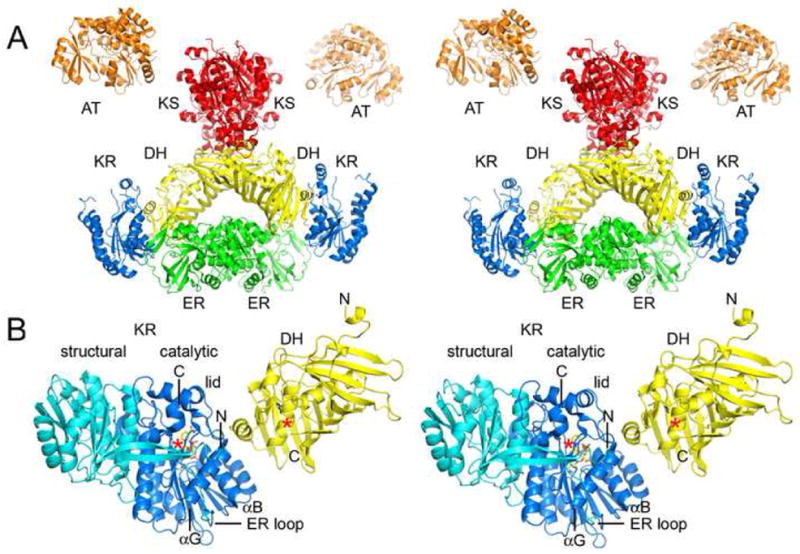
Domain Interfaces. A) Stereodiagram of the porcine FAS structure (obtained by Ban and coworkers by fitting the observed 4.5 Å resolution electron density map with solved type II enzymes)6. The structure indicates DH may contact KS, KR, and ER, and that DH monomers make contact across the twofold axis. B) PKS DH and KR domains (EryDH4 and EryKR1) were superposed on the equivalent porcine FAS domains to yield a more detailed model of the DH/KR interface. When ER is present, it inserts into the “ER loop” and makes contact across the twofold axis. The C-terminus of the DH fragment is ~10 Å from the N-terminus of the KR fragment. ACP is connected to the C-terminus of KR and would need to move to present polyketide intermediates to both active sites, which are separated by ~30 Å.
