Abstract
Ab responses in early life are low and short-lived; therefore, induction of protective immunity requires repeated vaccinations. One of the major limitations in early-life immunity is delayed maturation of follicular dendritic cells (FDCs), which play a central role in mediating the germinal center (GC) reaction leading to production of Ab-secreting cells (AbSCs). We assessed whether a nontoxic mutant of Escherichia coli heat-labile enterotoxin (LT-K63) and CpG1826 as model adjuvants could accelerate FDC maturation and immune response in neonatal mice, using a pneumococcal polysaccharide of serotype 1 conjugated to tetanus toxoid (Pnc1-TT) as a model vaccine. In neonatal NMRI mice, a single dose of Pnc1-TT coadministered with LT-K63 enhanced Pnc1-TT–induced GC reaction. In contrast, CpG1826 had no effect. Accordingly, LT-K63, but not CpG1826, accelerated the maturation of FDC networks, detected by FDC-M2+ staining, characteristic for adult-like FDCs. This coincided with migration of MOMA-1+ macrophages into the GCs that can enhance GC reaction and B cell activation. The FDC-M2+ FDC networks colocalized with enhanced expression of TNF-α, which is critical for the maintenance of mature FDCs and is poorly expressed in neonates. The accelerated maturation of FDC networks correlated with increased frequency and prolonged persistence of polysaccharide- and protein-specific IgG+ AbSCs in spleen and bone marrow. Our data show for the first time, to our knowledge, that an adjuvant (LT-K63) can overcome delayed maturation of FDCs in neonates, enhance the GC reaction, and prolong the persistence of vaccine-specific AbSCs in the BM. These properties are attractive for parenteral vaccination in early life.
Introduction
Immaturity of the immune system contributes to susceptibility to infectious diseases and poor vaccine responses in early life. Streptococcus pneumoniae causes annually 0.7–1.0 million deaths in children <5 y old (1). The incidence of pneumococcal diseases peaks at 3–18 mo (2) when maternal Abs have decreased (3) and the immune system is unable to respond to T cell-independent type 2 polysaccharides (PSs) (4). Protein conjugates of pneumococcal PSs (PPSs) are T cell dependent, immunogenic, and efficacious in infants (5). Induction of protective immunity in infancy requires two to three primary vaccinations; a toddler booster is essential to maintain protective Ab levels (6). Ab persistence is mediated by long-lived plasma cells in the bone marrow (BM) and generation of Ab-secreting cells (AbSCs) from memory B cells (7). High-affinity memory B cells are selected in germinal centers (GCs) in lymphoid organs (8), mediated by follicular dendritic cells (FDCs) retaining immune complexes (ICs) through FcRs and complement receptors (9). Cells involved in the initial deposition of ICs on FDCs include marginal metallophilic macrophages (MMMs) in spleen and subcapsular sinus macrophages of lymph nodes that both express CD169/MOMA-1 (10, 11). IC-bearing FDCs promote the GC formation (9), GC B cell survival (8), Ig class switching (9), somatic hypermutations, and selection of B cells with high-affinity receptors (8).
Establishment of GCs is delayed in human neonates (12), and Ab response is characterized by low somatic hypermutation frequency and poor selection of AbSCs in the GC during the first 6 mo of life (13). Accordingly, GC induction is limited in neonatal mice because of delayed FDC maturation, resulting in few AbSCs and weak Ab responses (14). The few plasmablasts generated in the spleen migrate to the BM but receive insufficient survival signal from a proliferation-inducing ligand (APRIL) to persist (15). All these factors contribute to the early-life characteristics of low vaccine-induced serum Ab levels and their rapid decline. Safe, effective adjuvants may overcome these limitations. Adjuvants have been shown to induce adult-like B and T cell activation, without being able to overcome the delayed FDC maturation in neonatal mice (12). Because a mature FDC network is critical for the optimal GC reaction, defining strategies that circumvent these limitations is of great importance to generate more effective vaccine formulations that would ultimately elicit balanced production of AbSCs and memory B cells, leading to sustained protective Ab production in childhood.
Using an early-life murine model of pneumococcal vaccination and challenge that reproduces the main features of human infant response to pneumococcal conjugate vaccines (16), we have reported that coadministration of the nontoxic adjuvant LT-K63 (nontoxic mutant of Escherichia coli heat-labile enterotoxin) (17, 18) with pneumococcal polysaccharide of serotype 1 conjugated to tetanus toxoid (Pnc1-TT) enhances protection against pneumococcal infection, correlating with enhanced PPS-specific Ab levels (16), avidity (19), and activation of B cells, T cells (20), and dendritic cells in spleen (21).
In this study, we assessed whether adjuvants could overcome the limited primary GC induction and FDC maturation in neonatal mice, using LT-K63 and the synthetic oligonucleotide CpG1826 as model adjuvants and Pnc1-TT as a model vaccine, and how that related to Ab responses and IgG+ AbSCs in spleen and BM. Neonatal (1 wk) and adult (6 wk) mice were immunized once with 0.5 μg Pnc1-TT alone or with 5 μg LT-K63 or 20 μg CpG1826. Spleens and BM were removed for analyses of GCs and AbSCs 14 and 10 d later, respectively, as their induction is age dependent and peaks at these time points after immunization with tetanus toxoid (TT) (14). Control mice received LT-K63 or saline.
Materials and Methods
Mice
Adult NMRI mice (M&B AS, Ry, Denmark) were kept with free access to commercial pelleted food and water, with regulated daylight, humidity, and temperature. Breeding cages were checked daily for births, and pups were kept with the mothers until weaning. The experiments were approved by the Experimental Animal Committee of Iceland.
Vaccine and adjuvants
PPS of serotype 1 (PPS-1) conjugated to TT (Pnc1-TT) (22) was provided by Sanofi Pasteur (Marcy l’Etoile, France). LT-K63 was produced by Novartis Vaccines and Diagnostics as described previously (23). CpG-ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was purchased from Oligos Etc. (Willsonville, OR).
Immunization
Neonatal (1 wk) and adult (6 wk) mice were immunized s.c. in the scapular girdle with 0.5 μg Pnc1-TT with or without 5 μg LT-K63 in 50 or 200 μl LT-K63 or saline, respectively. Blood was obtained from the tail vein 10 d (adult mice) or 14, 21, 23, 39, and 55 d (neonatal mice) after priming; serum was isolated and stored at −20°C. Spleens were removed and half was mounted in OCT, snap frozen, and kept at −70°C; the other half was used to numerate specific AbSCs.
ELISA
PPS-1– and TT-specific Abs (IgG) were measured by ELISA (24). Microtiter plates (MaxiSorp; Nunc AS, Roskilde, Denmark) were coated with 5 μg PPS-1/ml (American Type Culture Collection, Rockville, MD) PBS for 5 h at 37°C or 5 μg TT (Sanofi Pasteur) per milliliter 0.10 M carbonate buffer (pH 9.6) overnight at 4°C and blocked with PBS-Tween 20 and 1% BSA (Sigma). Serially diluted serum samples and standard neutralized by cell wall PSs (Statens Serum Institute, Copenhagen, Denmark) were incubated at room temperature for 2 h, followed by HRP goat anti-mouse Ab (Southern Biotechnology Associates, Birmingham, AL). The reaction was developed by 3,3′,5,5′-tetramethylbenzidine-substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD), stopped with 0.18 M H2SO4, and read at 450 nm in Titertek Multiscan Plus MK II spectrophotometer (ICN Flow Laboratories, Irvine, U.K.). Results were expressed as mean log ELISA units (EU)/ml ± SD, calculated from a standard curve (24).
Ab avidity
Avidity of IgG Abs was measured by ELISA with a kaliumthiocyanate (KSCN) elution step (19). Cell wall PS-neutralized standard and sera (1:50) were incubated in PPS-1– or TT-coated plates for 2 h. PBS-Tween 20 (100% binding) or KSCN dilutions (7.5–0.117 [M]) were incubated for 15 min to displace bound Abs. Remaining Abs were detected by alkaline phosphatase (ALP)-goat anti-mouse IgG (Southern Biotechnology Associates). The reaction was developed by paranitrophenylphosphate (Sigma) in diethanolamine buffer (pH 9.8), and absorbance was read at 405 nm. Results were expressed as avidity index (AI) = [M] KSCN displacing 50% of Abs.
Enumeration of AbSCs by ELISPOT
PPS-1– and TT-specific AbSC were enumerated by ELISPOT. MultiScreen High protein binding immobilon-P membrane plates (Millipore Corporation, Bedford, MA) were coated with 20 μg/ml PPS-1 or 10 μg/ml TT overnight at 37°C, blocked with complete RPMI 1640, serial dilutions of spleen cell (108cells/ml) in complete RPMI 1640 (Life Technologies BRL, Life Technologies, Paisley, U.K.) (20) were incubated for 5 h at 37°C, washed and incubated with ALP-goat anti-mouse IgG (Southern Biotechnology Associates) overnight at 4°C, and developed by 5-bromo-4-chloro-3-indolylphosphate and NBT in AP development buffer (Bio-Rad Labs, Hercules, CA). Spots were counted using a microscope (Zeiss, Oberkochen, Germany) and analyzed with KS ELISPOT (Zeiss).
Immunohistochemistry
Spleens were frozen in Tissue-Tek OCT (Sakura, Zouterwoude, the Netherlands) and cut into 7-μm cryosections at 4 levels, starting 700 μm into the tissue; the levels were separated by 210 μm, fixed in acetone for 10 min, and stored at −70°C. Four sections/spleen were stained with peanut agglutinin (PNA)-bio (Vector Laboratories, Burlingame, CA) that labels GC B cells followed by ALP-avidin (Mabtech AB, Nacka Strand, Sweden), to enumerate GCs and to enumerate follicles with IgG-HRP (switched) or IgM-HRP (nonswitched; Southern Biotechnology Associates). Mature FDCs were detected with mAbs to FDC-M2 (AMS Biotechnology Limited, Oxfordshire, U.K.), FDC-M1 (4C11; BD Pharmingen, San Diego, CA), and complement receptor 1 (CR1; 8C12; BD Pharmingen) followed by biotin-Ig (BD Pharmingen) and ALP- (Mabtech AB) or Texas Red-avidin (Jackson Immunoresearch Laboratories, Suffolk, U.K.). CXCL13 was detected with biotin-BLC (R&D Systems, Minneapolis, MN), metallophilic marginal macrophages with MOMA-1 (AbD Serotec, Düsseldorf, Germany), and C3-producing macrophages with MOMA-2 (AbD Serotec). TNF-α was detected with goat anti-mouse TNF-α (c-1351; Santa Cruz Biotechnology, Santa Cruz, CA) followed by Ig-Alexa Fluor 488 (Invitrogen, Eugene, OR). Nuclear counterstaining was done with DAPI (Invitrogen). Sections were photographed with a digital camera (AXIOCAM; Zeiss) using a microscope (Zeiss) equipped with ×10 and ×40 objectives and AxioImaging software (Birkerod, Denmark) for light and three-color immunofluorescence.
TNF-α levels in supernatant of spleen cells stimulated with TT in vitro
Three weeks after neonatal priming with Pnc1-TT with or without LT-K63, 107 spleen cells/ml in complete RPMI 1640 were incubated with or without TT (10 μg/ml) in 24-well plates (Nunc) at 37°C, 5% CO2 for 48 h (20). Supernatants (kept at −70°C) were incubated for 2 h in plates (MaxiSorp; Nunc), coated with anti-mouse TNF-α (R&D Systems), and blocked with PBS 1% BSA (Sigma). Biotin anti–TNF-α (R&D Systems) in PBS 1% BSA was incubated for 2 h and HRP-avidin (1:1000; BD Pharmingen) for 30 min. The reaction was developed as described earlier. TNF-α concentrations were calculated from a standard (R&D Systems) and expressed as pg/ml.
Statistical analysis
For comparison between groups and time points, Mann–Whitney rank sum test was used and Student t test when the data were normally distributed, using GraphPad Prism (GraphPad Software, La Jolla, CA). A p value <0.05 was considered statistically significant.
Results
Pneumococcal conjugate-induced GC formation in neonatal mice is enhanced by LT-K63
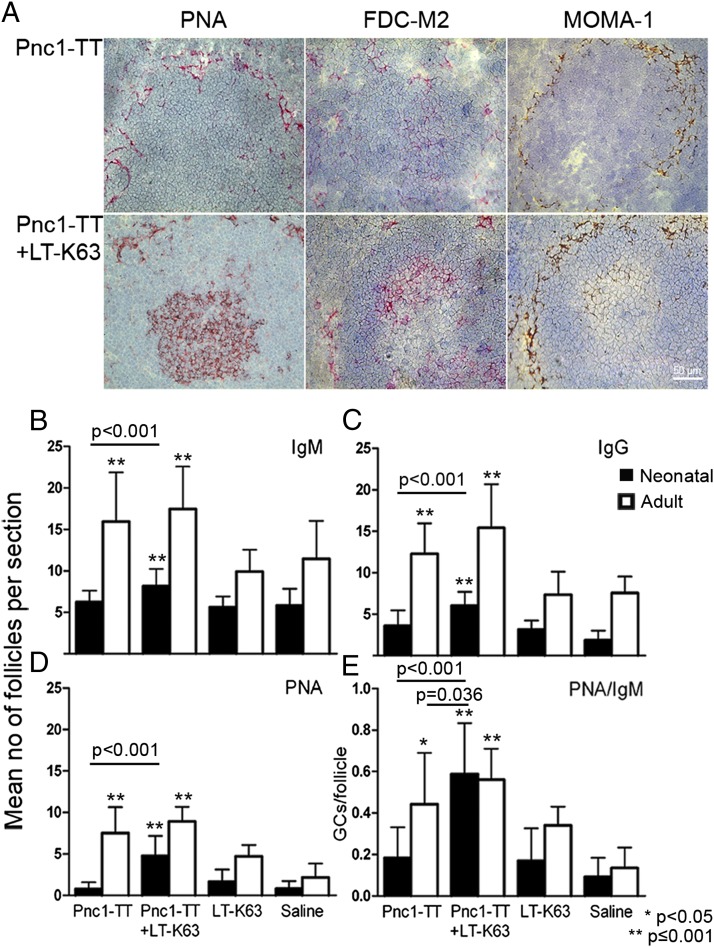
The effect of LT-K63 on Pnc1-TT–induced GC formation was studied by staining consecutive spleen sections with PNA, anti-IgM, and anti-IgG. Pnc1-TT induced limited GC reaction, shown by few weakly PNA+ follicles, and no difference in PNA+ GC numbers between mice immunized with Pnc1-TT or saline (Fig. 1A, 1E). LT-K63 enhanced the Pnc1-TT–induced GC reaction in neonatal mice significantly, leading to higher PNA+ GC frequencies than in Pnc1-TT–immunized or control mice (LT-K63 or saline; Fig. 1D). Despite the increase of GC numbers induced by LT-K63 in neonatal mice, they were still fewer than in adult mice (Pnc1-TT or Pnc1-TT+LT-K63: p < 0.001) (Fig. 1D). Importantly, the GCs were more structured and organized in neonatal mice that received LT-K63 with Pnc1-TT (Fig. 1A, Supplemental Fig. 1). The numbers of unswitched IgM+ and switched IgG+ follicles were significantly enhanced by LT-K63 in neonatal mice compared with Pnc1-TT–immunized and control mice (Fig. 1B, 1C). Still, neonates immunized with Pnc1-TT+LT-K63 had fewer IgM+ and IgG+ follicles than adult mice immunized with Pnc1-TT (IgM: p < 0.001, IgG: p < 0.001) or Pnc1-TT+LT-K63 (IgM: p < 0.001, IgG: p < 0.001). The ratio of PNA+ GCs/IgM+ follicles was calculated for each spleen section to adjust for size difference. The GC/follicle ratio was significantly higher in neonatal mice immunized with Pnc1-TT+LT-K63 than Pnc1-TT. The age-related difference was reduced by LT-K63, as adult and neonatal mice immunized with Pnc1-TT+LT-K63 had comparable GC/follicle ratios, and neonatal mice immunized with Pnc1-TT+LT-K63 had a significantly higher ratio than Pnc1-TT–immunized adult mice (Fig. 1E). These results demonstrate that LT-K63 can circumvent limited vaccine-induced GC formation in neonatal mice.
FIGURE 1.
LT-K63 overcomes delayed vaccine-induced GC reaction because of accelerated maturation of FDC network clusters in neonatal mice. (A) PNA- (left), FDC-M2– (middle), and MOMA-1 (right)–stained spleen sections from neonatal mice immunized with 0.5 μg Pnc1-TT (top panel) or 0.5 μg Pnc1-TT with 5 μg LT-K63 (lower panel). Spleens were removed 14 or 10 d after priming of neonatal and adult mice, respectively, when AbSCs and GCs in spleen have been shown to peak (14). Half of the spleen was snap frozen, serial 7-μm cryosection sections were cut at four levels, starting 700 μm into the tissue, and each level was separated by 210 μm. One representative section from each group is shown. Original magnification ×40. Scale bar, 50 μm. (B–E) Consecutive section from all four levels were stained with (B) anti-IgM, (C) anti-IgG, (D) PNA, and results (mean ± SD) are shown for each group of neonatal (filled bars) and adult (open bars) mice. (E) Mean GC/follicle ratio was calculated for each spleen at all four levels to adjust for age difference in spleen size, and results (mean ± SD) are shown for each group. Statistical difference between test groups and controls (LT-K63 and saline-immunized mice) are shown; *p < 0.05, **p ≤ 0.001. Results in (A)–(E) are from three experiments for neonatal mice (n = 24 per group, except for LT-K63–injected mice n = 16) and two experiments for adult mice (n = 16 per group, except for LT-K63–injected mice n = 8), with eight mice per group in each experiment.
LT-K63 enhances maturation of FDC network clusters in Pnc1-TT–immunized neonatal mice
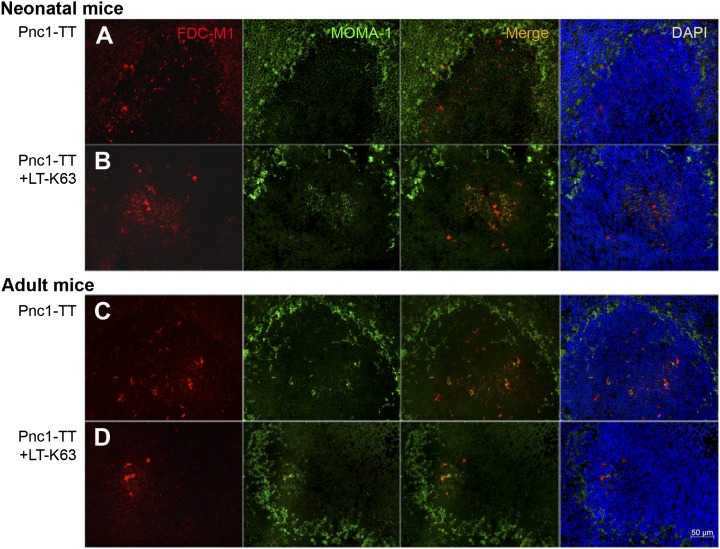
To investigate whether LT-K63 mediated enhanced GCs formation through its effects on the immature FDCs, we stained cryosections for markers characterizing mature FDC networks; FDC-M2, which reacts with C4 fragment associated with ICs (25); FDC-M1 identifying Mfge8, which is secreted by FDCs and mediates engulfment of apoptotic GC B cells by tingible-body macrophages (26), and 8C12 recognizing the ligand-binding site of CR1/CD35. Fourteen days after Pnc1-TT immunization, neonatal mice had hardly any FDC-M2+ FDC clusters (Fig. 1A), whereas those that received Pnc1-TT+LT-K63 had similar FDC-M2+ FDC staining pattern as in adult mice immunized with Pnc1-TT with or without LT-K63 (Fig. 1A, Supplemental Fig. 1), suggesting that LT-K63 induced their migration into the GCs. FDC-M1 staining was similar in neonatal and adult mice immunized with Pnc1-TT with or without LT-K63 (Fig. 2), and CR1 expression in splenic follicles was comparable regardless of whether neonatal mice received LT-K63 (Supplemental Fig. 2). Neonatal mice primed with Pnc1-TT with or without LT-K63 expressed the B cell chemoattractant CXCL13 (Supplemental Fig. 2) found on FDCs and follicular stromal cells (27).
FIGURE 2.
LT-K63 enhanced the colocalization of FDC-M1+ FDC networks and migrated MOMA-1+ MMMs in activated GCs in mice immunized as neonates. To detect FDCs or MMMs, we stained spleen sections with FDC-M1 (red) or MOMA-1 (green), respectively, or DAPI to visualize the nuclei (blue), 14 or 10 d after priming of neonatal (A, B) and adult (C, D) mice with 0.5 μg Pnc1-TT (A, C) or 0.5 μg Pnc1-TT with 5 μg LT-K63 (B, D). Seven-micrometer sections were cut from four levels in the spleen; the first started 700 μm into the tissue, and levels were separated by 210 μm. One representative section from each group is shown. Original magnification ×40. Scale bar, 50 μm. Results shown in (A)–(D) are from two experiments for neonatal and adult mice (n = 16 per group), with eight mice per group in each experiment.
Thus, the effects of LT-K63 were not associated with enhanced expression of FDC-M1, CR1, or CXCL13 by FDCs or stromal cells, but with enhanced FDC-M2 expression, reflecting enhanced FDC maturation and capacity to retain ICs, and promoting the GC reaction. LT-K63, to our knowledge, is the first adjuvant shown to overcome the delayed maturation of FDC networks in neonates.
LT-K63 induces migration of MOMA-1+ MMMs into activated GCs in neonatal mice
MMMs that line the inner layer of the marginal zone (MZ) (28) express high levels of MOMA-1, have low phagocytic capacity (10, 29), and migrate into the follicles in response to stimulation (10). Staining with MOMA-1 was performed to study the effects of LT-K63 on MMMs and with MOMA-2 to study the effects on MOMA-2+ macrophages, the major producers of C3 (30), which is important in activation of PS-specific B cells (31). Low serum C3 level is associated with poor Ab response to PS in newborn mice and infants (32, 33). In neonatal mice immunized with Pnc1-TT with or without LT-K63, staining of MOMA-1+ cells was observed in the inner layer of the MZ, slightly enhanced by LT-K63 (Figs. 1, 2A, 2B), to a similar intensity as observed in adult mice (Fig. 2C, 2D, Supplemental Fig. 1). LT-K63 enhanced the migration of MOMA-1+ cells into activated GCs in neonates colocalizing with FDC-M2+ and FDC-M1+ clusters, similar to that observed in adult mice immunized with Pnc1-TT with or without LT-K63 (Figs. 1A, 2, Supplemental Fig. 1). MOMA-2 staining in the white pulp and MZ was similar in neonatal mice immunized with Pnc1-TT with or without LT-K63 (Supplemental Fig. 2).
These results demonstrate that in neonates, LT-K63 stimulates migration of MMMs into activated GCs, consistent with enhanced deposition of ICs on FDC-M2+ FDC clusters (Fig. 1A, Supplemental Fig. 1).
LT-K63 enhances TNF-α expression in spleens of Pnc1-TT–primed neonatal mice
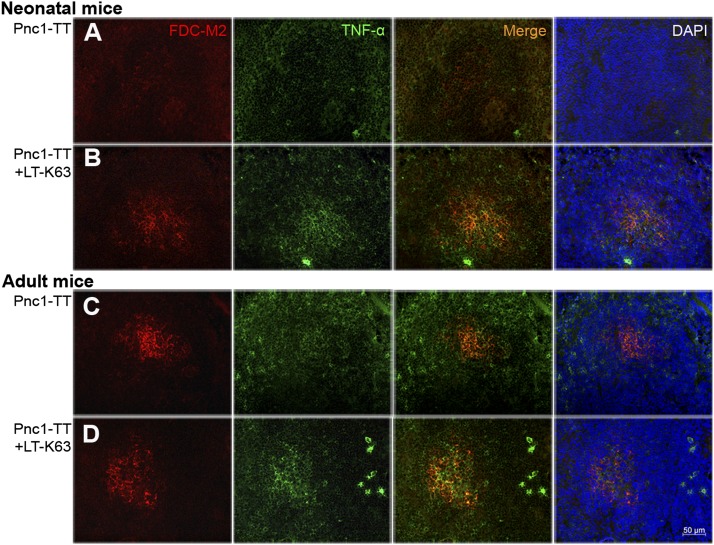
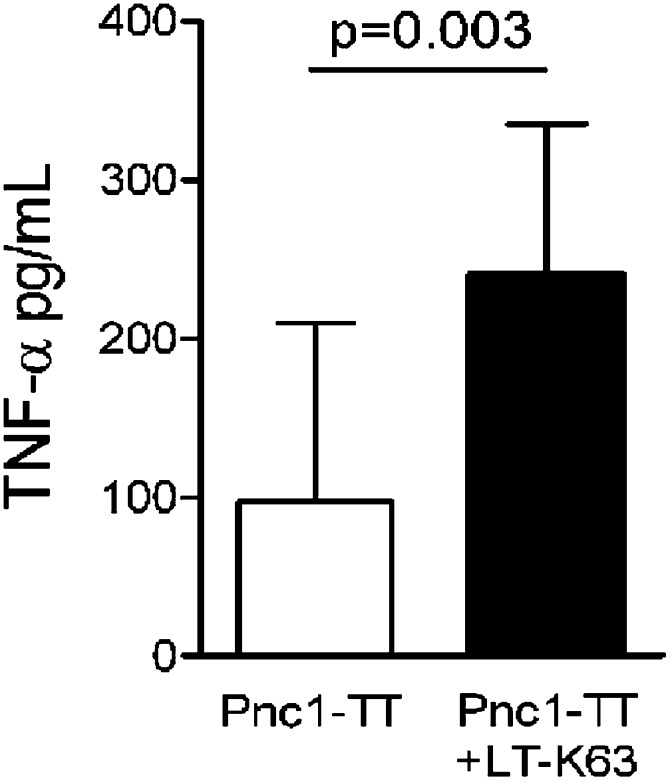
Signaling by TNF-α is required for FDC development (34), and TNF-α expression is delayed in early life (35). To investigate whether neonatal maturation of FDC network accelerated by LT-K63 was associated with increased TNF-α expression, we stained spleen sections with anti–TNF-α. Fourteen days after Pnc1-TT immunization, neonatal mice had hardly any detectable TNF-α in spleen (Fig. 3A). In contrast, mice immunized with Pnc1-TT+LT-K63 showed TNF-α staining that colocalized with FDC-M2+ cells in GCs, similar to adult mice (Fig. 3). For further validation, spleen cells obtained 3 wk after neonatal immunization were stimulated with TT (carrier protein of Pnc1-TT) for 48 h, and TNF-α was measured in the supernatants. Despite high individual variation observed after one neonatal immunization, TNF-α levels were significantly higher in mice immunized with Pnc1-TT+LT-K63 than Pnc1-TT (Fig. 4). These results demonstrate that LT-K63 is able to enhance delayed TNF-α production in the neonatal spleen that parallels with accelerated maturation of FDC networks.
FIGURE 3.
LT-K63 enhanced TNF-α expression that colocalized with FDC-M2 staining in mice immunized with Pnc1-TT as neonates. Spleen sections were stained with FDC-M2 (red), Ab to TNF-α (green), or DAPI to visualize the nuclei (blue) 14 or 10 d after priming of neonatal (A, B) and adult (C, D) mice with 0.5 μg Pnc1-TT (A, C) or 0.5 μg Pnc1-TT+5 μg LT-K63 (B, D), when induction of GCs in spleen peaks in neonatal versus adult mice (14). Seven-micrometer sections were prepared from four different levels, the first started 700 μm into the spleen and the levels were separated by 210 μm. One representative section from each group is shown. Original magnification ×40. Scale bar, 50 μm. Results shown in (A)–(D) are from two experiments for neonatal and adult mice (n = 16 per group), with eight mice per group in each experiment.
FIGURE 4.
LT-K63 enhanced the TNF-α levels in cell culture supernatants after in vitro TT stimulation of spleen cells from mice immunized with Pnc1-TT as neonates. Spleen cells isolated 3 wk after priming of neonatal mice with Pnc1-TT (open column) or Pnc1-TT+LT-K63 (filled column) were stimulated with or without TT (10 μg/ml) for 48 h (20). TNF-α in the supernatants was measured by ELISA. The results are shown as pg TNF-α/ml (mean ± SD) in TT-stimulated cultures after subtracting TNF-α levels in unstimulated cultures. Student t test was applied, and p < 0.05 was considered statistically significant. Results shown are from two experiments for neonatal mice (n = 14 per group), with seven mice per group in each experiment.
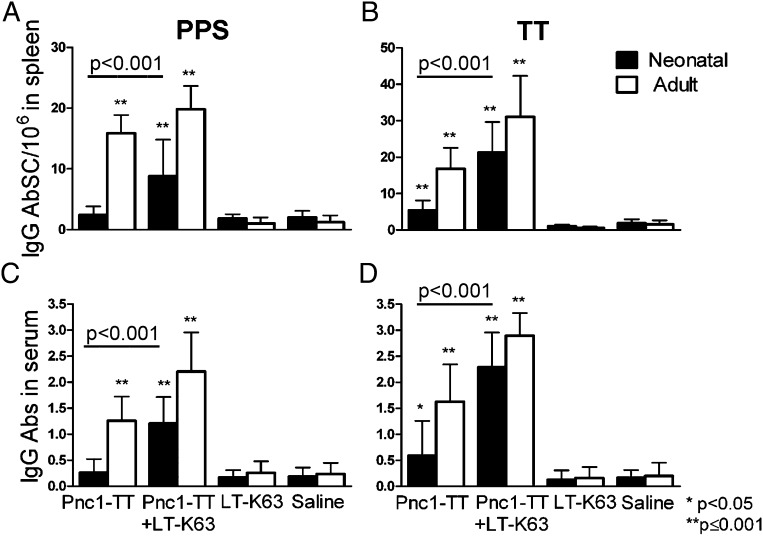
LT-K63 enhances primary induction of PS and protein carrier-specific AbSCs in neonatal mice
We explored whether the LT-K63–enhanced GC reaction in neonates was associated with enhanced primary induction of AbSCs. PPS-1– and TT-specific IgG+ AbSCs in spleen were enumerated by ELISPOT 14 or 10 d after immunization of neonatal and adult mice, respectively. In neonatal mice immunized with Pnc1-TT, the frequency of PPS-1–specific AbSCs was comparable with that in controls (Fig. 5A), but TT-specific AbSC frequency was higher (Fig. 5B). Thus, limited GC formation in neonates after Pnc1-TT immunization parallels the low frequency of PPS-1–specific AbSCs in the spleen. Compared with Pnc1-TT alone, coadministration of LT-K63 significantly enhanced the frequency of PPS-1– and TT-specific AbSCs (Fig. 5A, 5B) reflected by increased serum IgG anti–PPS-1 and anti-TT (Fig. 5C, 5D). The frequency of PPS-1–specific AbSCs was lower in neonatal than in adult mice (p < 0.001), but TT-specific AbSCs was not (p = 0.119; Fig. 5A, 5B). These results show a clear association between impaired GC reaction and limited AbSC induction after Pnc1-TT priming in early life that was more pronounced for the PS part of the vaccine. Furthermore, the accelerated maturation of FDC networks by LT-K63 parallels increased primary induction of AbSCs in spleen.
FIGURE 5.
LT-K63 increased the frequency of Pnc1-TT–specific AbSCs. PPS-1– and TT-specific IgG+ AbSCs measured by ELISPOT, shown as number of spots (mean ± SD) per 106 cells (A, B), and PPS-1– and TT-specific IgG levels (mean EU/ml ± SD) in serum measured by ELISA (C, D). Results are shown for neonatal (filled bars) and adult (open bars) mice immunized with 0.5 μg Pnc1-TT with or without 5 μg LT-K63, LT-K63, or saline. Spleens were removed 14 or 10 d after priming, when GCs and AbSCs peak in neonatal and adult mice, respectively (14), and single-cell suspension was prepared from half of the spleen. Statistical difference between test groups and controls (LT-K63– and saline-immunized mice) are shown; *p < 0.05, **p ≤ 0.001. Results in (A)–(D) are from three experiments for neonatal mice (n = 24 per group, except for LT-K63 injected mice n = 16) and two experiments for adult mice (n = 16 per group, except for LT-K63–injected mice n = 8), with eight mice per group in each experiment.
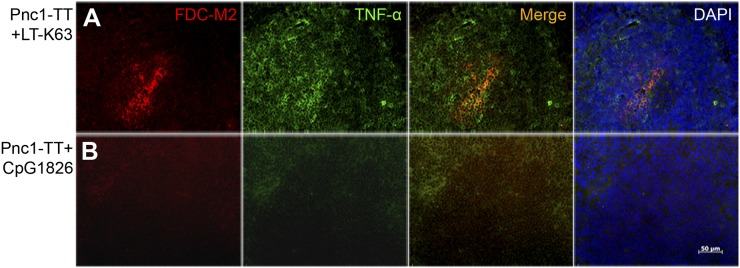
CpG1826 does not enhance maturation of early-life FDCs or TNF-α expression
CpG1826, the optimal murine CpG-ODN motif, enhanced TT-specific Abs and AbSCs in neonatal mice immunized with TT absorbed in AL(OH)3, but failed to overcome limited GC induction (36) and FDC maturation (14). We compared the effects of LT-K63 and CpG1826 on Pnc1-TT–induced GC formation and AbSCs, and found LT-K63 to enhance GC formation at days 12, 14, and 16, whereas CpG1826 had no effect, shown by few weakly PNA+ follicles (Supplemental Fig. 3), similar to mice immunized with Pnc1-TT or saline (Fig. 1A). Only neonatal mice that received Pnc1-TT with LT-K63, but not with CpG1826, showed increased FDC maturation at all time points (Fig. 6, Supplemental Fig. 3). In contrast with mice immunized with Pnc1-TT+LT-K63, mice immunized with Pnc1-TT+CpG1826 had hardly any detectable TNF-α in spleen at days 12 and 14 (Fig. 6B), although a weak TNF-α staining was detected in Pnc1-TT+CpG1826 immunized mice at day 16 (Supplemental Fig. 3). In contrast, both LT-K63 and CpG1826 enhanced equally the frequency of PPS-1– and TT-specific IgG+ AbSCs in spleen and IgG Abs in serum at all time points (Supplemental Fig. 4). These results further confirm that so far, LT-K63 is the only adjuvant shown to overcome delayed maturation of FDCs that parallels enhanced TNF-α expression in spleen.
FIGURE 6.
CpG1826 does not overcome delayed maturation of FDCs and lack of TNF-α expression in GCs in early life. Spleen sections were stained with FDC-M2 (red), Ab to TNF-α (green), or DAPI to visualize the nuclei (blue) 12 d after priming of neonatal mice with (A) 0.5 μg Pnc1-TT+5 μg LT-K63 or (B) 0.5 μg Pnc1-TT+20 μg CpG1826. Seven-micrometer sections were cut at four different levels; the first started 700 μm into the spleen, and levels were separated by 210 μm. One representative section from each group is shown. Original magnification ×40. Scale bar, 50 μm. Results shown in (A) and (B) are from two experiments in neonatal mice with nine mice per group.
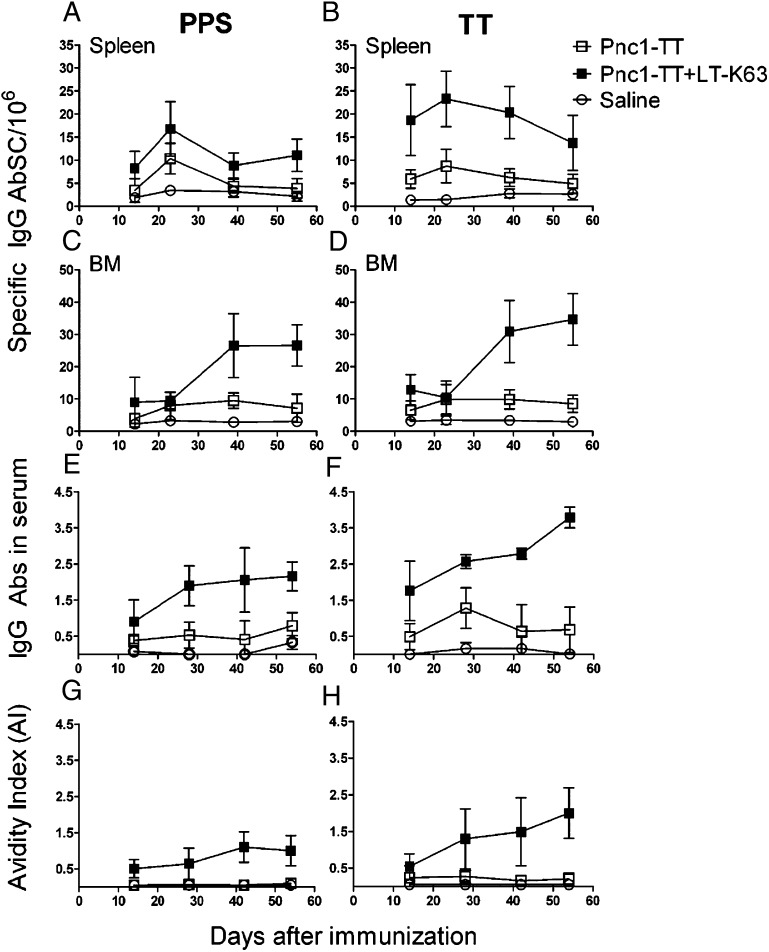
LT-K63 enhances long-term persistence of early-life AbSCs in spleen and BM
The effects of LT-K63 on long-term persistence of vaccine-specific AbSCs in neonatal mice were evaluated by enumeration of IgG+ AbSCs in spleen and BM ex vivo by ELISPOT up to 8 wk after one neonatal immunization. At day 14, LT-K63 enhanced the frequency in spleen (Fig. 7A, 7B) and BM (Fig. 7C, 7D) of PPS-1– (spleen: p = 0.003; BM: p = 0.0025) and TT-specific (spleen: p < 0.001; BM: p = 0.010) AbSCs, indicating enhanced AbSC output of the GC reaction resulting in more vaccine-specific AbSCs that homed to the BM. Similarly, LT-K63 increased the frequency of PPS-1– (p = 0.006) and TT-specific (p < 0.001) AbSCs at day 23 in spleen (Fig. 7A, 7B) and BM (p = 0.049) (Fig. 7C), but TT-specific AbSCs were comparable (Fig. 7D). At day 39, splenic AbSCs (PPS-1: p < 0.001; TT: p = 0.049) had decreased from day 23 in mice immunized with Pnc1-TT with or without LT-K63 (PPS-1: p = 0.002), whereas increased IgG+ PPS-1– and TT- specific AbSCs were observed in BM of neonatal mice immunized with Pnc1-TT+LT-K63 (p < 0.001), but not if immunized with Pnc1-TT (PPS-1: p = 0.076; TT: p = 0.844). Thus, the frequency of PPS-1– and TT-specific AbSCs in spleen and BM was higher in mice immunized with Pnc1-TT with LT-K63 than without (p < 0.001). This difference in AbSCs persisted in the BM at day 55 (p < 0.001; Fig. 7C, 7D). Accordingly, PPS-1– and TT-specific IgG levels (Fig. 7E, 7F) and avidity (Fig. 7G, 7H) were increased by LT-K63 and continued to increase up to 8 wk after immunization. The results clearly demonstrate the ability of LT-K63 to enhance primary vaccine-specific AbSC response in spleens of neonates and prolong their persistence in spleen and BM.
FIGURE 7.
LT-K63 enhanced the induction and long-term persistence of vaccine-specific AbSCs. PPS-1–specific (left) and TT-specific (right) IgG+ AbSCs in spleen (A, B) and BM (C, D) measured by ELISPOT, and serum IgG Ab levels (E, F) and AIs (G, H) measured by ELISA at days 14, 23, 39, and 55 after priming of neonatal mice with Pnc1-TT (□), Pnc1-TT+LT-K63 (▪), or saline (○). The results are from two experiments, expressed as number of spots/106 cells (mean ± SD), IgG levels (mean EU/ml ± SD), and AI (mean AI ± SD) in 12 mice/group for each time point.
Discussion
This study demonstrates for the first time, to our knowledge, that delayed FDC maturation and limited GC induction in early life can be overcome by the adjuvant LT-K63, which enhances primary induction of PPS-1– and TT-specific AbSCs and prolongs their persistence in the BM, explaining prolonged protective Ab levels reported (16, 19). Our results also show that this capacity is not common to all adjuvants because CpG-1826, the prototype TLR-9 agonist, does not exhibit these effects.
Safe and effective adjuvants could improve priming conditions in early life, converting weak and delayed IgG Ab responses of short duration into rapid and persistent protective immunity. Alum, the only adjuvant currently licensed for human infant vaccines, is relatively poor at inducing protective, adult-like immune responses (12).
Unlike wild-type LT (37), LT-K63 has no detectable ADP ribosylating activity or toxicity in vitro or in vivo (17, 18). Mucosal application of LT-K63 with an inactivated influenza vaccine resulted in protective Ab response and a good safety profile (38). In a second study, two individuals experienced transient facial nerve palsy, causing reconsideration of intranasal application of this family of molecules (39).
In this study, we demonstrate that s.c. administration of LT-K63 markedly enhances Pnc1-TT–induced GC formation, associated with enhanced maturation of FDC-M2+ FDCs in GCs. Mature FDCs express CR1 that retain ICs (9) and secrete Mfge8 (FDC-M1) that licenses engulfment of apoptotic GC B cells by tingible-body macrophages (26). CR1 and FDC-M1 were not limiting for the FDC maturation in our study, in agreement with FDC-M1 and CR1 expression demonstrated in 3- and 7-d-old BALB/c mice, respectively (40). Movement of FDCs into follicular clusters increases CXCL13 expression that attracts B cells (41), and CXCL13 is detected in follicles of 14-d-old mice (14). We found no difference in CXCL13 staining between neonatal mice immunized with Pnc1-TT with or without LT-K63. Therefore, the main limiting factor in neonatal GC reaction seems to be poor retention of ICs by the FDCs, associated with weak FDC-M2+ expression (25), which the adjuvant LT-K63 was able to accelerate. In agreement with our results, addition of CpG1826 to a TT+Alum vaccine formulation neither overcomes delayed GC induction nor limits FDC maturation in neonates (14, 36). Increased IC deposition enhances the selection of B cells and production of memory B cells with high affinity for the Ag retained by the FDCs (8). Accordingly, coadministration of LT-K63 with one neonatal dose of Pnc1-TT increases PPS-1–specific IgG avidity and levels that persist above protective levels against pneumococcal bacteremia (1.0 log EU/ml) and lung infection (2.5 log EU/ml) (16) for at least 12 wk (19). Early after immunization, CpG1826 was able to enhance vaccine-specific Abs and AbSCs in spleen. We reported that LT-K63 and CpG-ODN work through different mechanisms in enhancing Pnc1-TT–induced responses in early life. Both adjuvants enhanced neonatal B cell activation, with comparable induction of protective Abs (20), but only LT-K63 improved T cell activation (20, 42). Both adjuvants enhanced the expression of MHC class II and costimulatory molecules on neonatal DCs in vitro (21), but unlike LT-K63, CpG-ODN did not enhance T cell priming (20, 42). CpG1826 enhances IFN-γ response in adult (43), but not neonatal, mice (42). CpG1826-mediated TLR9 signaling in vivo, but not in vitro, was recently shown to induce high IL-10 secretion by neonatal CD5+ B cells that prevented optimal IL-12 secretion by neonatal DCs, thus blocking their Th1-priming capacity (44). TNF-α induces FDC proliferation and network formation (45), and TNF-α signaling through TNFRp55 leads to migration of FDC precursors into the B cell follicles in TNF knockout mice (41), in agreement with our results in neonatal mice receiving Pnc1-TT+LT-K63. We show that TNF-α staining colocalizing with FDC-M2+ cells in GCs is increased in mice primed with Pnc1-TT+LT-K63 compared with mice primed with Pnc1-TT with or without CpG1826, and TT-induced TNF-α secretion by spleen cells was also increased. Accordingly, LT-K63 enhances NF-κB translocation in macrophages of adult mice (18) and increases TNF-α production by CD4+ T cells in vivo (46). Although CpG-ODNs are strong TNF-α inducers in adult mice (43), CpG1826 failed to overcome the delayed TNF-α production in our neonatal murine model, in contrast with LT-K63. This confirms that one of the mechanisms by which LT-K63 accelerates the maturation of FDCs is through enhanced TNF-α production.
Native PPSs rapidly localize on MZ B cells, whereas after pneumococcal conjugate immunization, PPSs localize in proximity of MMMs (47). The migratory ability of MOMA-1+ MMMs into developing GCs (10) is required for the initiation of immune response to T cell-dependent (48) and type 2 Ags (28). Subcapsular sinus MOMA-1+ macrophages capture ICs and deliver to FDCs (49) or follicular B cells (11) that act as transporters to the FDCs. Migration of MOMA-1+ MMMs into activated GCs, colocalizing with FDC-M2+ and FDC-M1+ FDC network clusters, was observed in neonatal mice immunized with Pnc1-TT+LT-K63. The follicular migration of MMMs is dependent on LT-β receptor expression (29, 45) that requires LT-α1β2 expression by B cells (27), and both are delayed in early life (50). The presence of MOMA-1+ macrophages increases B cell activation (51), magnitude, and quality of the GC response (29). Accordingly, migration of MOMA-1+ MMMs into GCs parallels with increased avidity of PPS-1– and TT-specific Abs over a long time.
The effect of LT-K63 on Pnc1-TT–induced GC reaction and FDC network maturation correlated with increased generation of primary Pnc1-TT–specific IgG+ AbSCs in neonatal mice and prolonged their persistence in both spleen and BM. Furthermore, increase of Pnc1-TT–specific IgG+ AbSCs in BM induced by LT-K63 occurred more rapidly than reported for CpG1826, which had limited effect on the BM AbSC pool (36). AbSC persistence depends on survival niches in spleen, lymph nodes, and BM (7). Factors known to influence growth, maturation, and survival of plasmablasts include IL-6, CXCL12, TNF-α (52), BAFF, and APRIL (15, 53). Expression of TNF-α in spleen (35) and APRIL in BM (15) is poor during the neonatal period. Maintenance of differentiated FDCs requires continuous stimulation by membrane-bound TNF-α and LT-α1β2 on B cells (45). Thus, access of late-arriving B cells to Ags on FDCs prolongs the GC reaction (54) and increases the GC output of AbSCs. LT-K63 corrected the poor persistence of PPS-1– and TT-specific AbSCs in neonates, possibly through prolonged output of AbSCs from the GC reactions because of enhanced expression of receptors crucial for survival and continuous homing to the BM. Whether LT-K63 increases survival signals in the BM is unknown.
Taken together, LT-K63 is the first adjuvant, to our knowledge, shown to overcome delayed FDC network maturation in early life and enhance the induction and magnitude of GC reaction that correlates with increased generation of PS- and protein-specific IgG+ AbSCs and their prolonged persistence in the BM. Further studies are needed to dissect the complex cellular interactions and molecular pathways involved, which may advance the development of improved parenteral vaccination strategies for human neonates and infants.
Supplementary Material
Acknowledgments
We thank Dr. Emanuelle Trannoy (Sanofi Pasteur, France) for providing Pnc1-TT and Dr. Maren Henneken (Department of Immunology, Landspitali, The National University Hospital of Iceland) for helpful advice regarding the immunohistochemistry.
This work was supported by the Icelandic Research Fund for Graduate Students, the Icelandic Research Fund, the Eimskip Fund of the University of Iceland, the Landspitali University Hospital Research Fund, and the University of Iceland Research Fund.
The online version of this article contains supplemental material.
- AbSC
- Ab-secreting cell
- AI
- avidity index
- ALP
- alkaline phosphatase
- APRIL
- a proliferation-inducing ligand
- BM
- bone marrow
- CR1
- complement receptor 1
- EU
- ELISA unit
- FDC
- follicular dendritic cell
- GC
- germinal center
- IC
- immune complex
- KSCN
- kaliumthiocyanate
- LT-K63
- nontoxic mutant of Escherichia coli heat-labile enterotoxin
- MMM
- marginal metallophilic macrophage
- MZ
- marginal zone
- PNA
- peanut agglutinin
- Pnc1-TT
- pneumococcal polysaccharide of serotype 1 conjugated to tetanus toxoid
- PPS
- pneumococcal polysaccharide
- PPS-1
- PPS of serotype 1
- PS
- polysaccharide
- TT
- tetanus toxoid.
Disclosures
G.D.G. is a full-time employee of Novartis Vaccines and Diagnostics. I.J. has received research grants that exceed $5000 per year from the Icelandic Research Fund, Landspitali University Hospital Research Fund, the University of Iceland Research Fund, and the European Commission Sixth Framework Programme for Research and Technological Development Life Sciences during the last five years. The other authors have no financial conflicts of interest.
References
- 1.WHO 2007. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly. Epidemiol. Rec. 82: 93–104 [PubMed] [Google Scholar]
- 2.Scott J. A. 2007. The preventable burden of pneumococcal disease in the developing world. Vaccine 25: 2398–2405 [DOI] [PubMed] [Google Scholar]
- 3.Shahid N. S., Steinhoff M. C., Hoque S. S., Begum T., Thompson C., Siber G. R. 1995. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet 346: 1252–1257 [DOI] [PubMed] [Google Scholar]
- 4.Douglas R. M., Paton J. C., Duncan S. J., Hansman D. J. 1983. Antibody response to pneumococcal vaccination in children younger than five years of age. J. Infect. Dis. 148: 131–137 [DOI] [PubMed] [Google Scholar]
- 5.Black S., Shinefield H., Fireman B., Lewis E., Ray P., Hansen J. R., Elvin L., Ensor K. M., Hackell J., Siber G. R., et al. Northern California Kaiser Permanente Vaccine Study Center Group 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19: 187–195 [DOI] [PubMed] [Google Scholar]
- 6.Sigurdardottir S. T., Davidsdottir K., Arason V. A., Jonsdottir O., Laudat F., Gruber W. C., Jonsdottir I. 2008. Safety and immunogenicity of CRM197-conjugated pneumococcal-meningococcal C combination vaccine (9vPnC-MnCC) whether given in two or three primary doses. Vaccine 26: 4178–4186 [DOI] [PubMed] [Google Scholar]
- 7.Radbruch A., Muehlinghaus G., Luger E. O., Inamine A., Smith K. G., Dörner T., Hiepe F. 2006. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6: 741–750 [DOI] [PubMed] [Google Scholar]
- 8.Wu Y., Sukumar S., El Shikh M. E., Best A. M., Szakal A. K., Tew J. G. 2008. Immune complex-bearing follicular dendritic cells deliver a late antigenic signal that promotes somatic hypermutation. J. Immunol. 180: 281–290 [DOI] [PubMed] [Google Scholar]
- 9.Aydar Y., Sukumar S., Szakal A. K., Tew J. G. 2005. The influence of immune complex-bearing follicular dendritic cells on the IgM response, Ig class switching, and production of high affinity IgG. J. Immunol. 174: 5358–5366 [DOI] [PubMed] [Google Scholar]
- 10.Groeneveld P. H., Erich T., Kraal G. 1986. The differential effects of bacterial lipopolysaccharide (LPS) on splenic non-lymphoid cells demonstrated by monoclonal antibodies. Immunology 58: 285–290 [PMC free article] [PubMed] [Google Scholar]
- 11.Phan T. G., Grigorova I., Okada T., Cyster J. G. 2007. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8: 992–1000 [DOI] [PubMed] [Google Scholar]
- 12.Siegrist C. A., Aspinall R. 2009. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9: 185–194 [DOI] [PubMed] [Google Scholar]
- 13.Rajewsky K. 1996. Clonal selection and learning in the antibody system. Nature 381: 751–758 [DOI] [PubMed] [Google Scholar]
- 14.Pihlgren M., Tougne C., Bozzotti P., Fulurija A., Duchosal M. A., Lambert P. H., Siegrist C. A. 2003. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 170: 2824–2832 [DOI] [PubMed] [Google Scholar]
- 15.Belnoue E., Pihlgren M., McGaha T. L., Tougne C., Rochat A. F., Bossen C., Schneider P., Huard B., Lambert P. H., Siegrist C. A. 2008. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111: 2755–2764 [DOI] [PubMed] [Google Scholar]
- 16.Jakobsen H., Bjarnarson S., Del Giudice G., Moreau M., Siegrist C. A., Jonsdottir I. 2002. Intranasal immunization with pneumococcal conjugate vaccines with LT-K63, a nontoxic mutant of heat-Labile enterotoxin, as adjuvant rapidly induces protective immunity against lethal pneumococcal infections in neonatal mice. Infect. Immun. 70: 1443–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizza M., Fontana M. R., Giuliani M. M., Domenighini M., Magagnoli C., Giannelli V., Nucci D., Hol W., Manetti R., Rappuoli R. 1994. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. J. Exp. Med. 180: 2147–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan E. J., McNeela E., Pizza M., Rappuoli R., O’Neill L., Mills K. H. 2000. Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro- and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity. J. Immunol. 165: 5750–5759 [DOI] [PubMed] [Google Scholar]
- 19.Bjarnarson S. P., Jakobsen H., Del Giudice G., Trannoy E., Siegrist C. A., Jonsdottir I. 2005. The advantage of mucosal immunization for polysaccharide-specific memory responses in early life. Eur. J. Immunol. 35: 1037–1045 [DOI] [PubMed] [Google Scholar]
- 20.Olafsdottir T. A., Hannesdottir S. G., Giudice G. D., Trannoy E., Jonsdottir I. 2007. Effects of LT-K63 and CpG2006 on phenotype and function of murine neonatal lymphoid cells. Scand. J. Immunol. 66: 426–434 [DOI] [PubMed] [Google Scholar]
- 21.Hannesdottir S. G., Olafsdottir T. A., Giudice G. D., Jonsdottir I. 2008. Adjuvants LT-K63 and CpG enhance the activation of dendritic cells in neonatal mice. Scand. J. Immunol. 68: 469–475 [DOI] [PubMed] [Google Scholar]
- 22.Szu S. C., Taylor D. N., Trofa A. C., Clements J. D., Shiloach J., Sadoff J. C., Bryla D. A., Robbins J. B. 1994. Laboratory and preliminary clinical characterization of Vi capsular polysaccharide-protein conjugate vaccines. Infect. Immun. 62: 4440–4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuliani M. M., Del Giudice G., Giannelli V., Dougan G., Douce G., Rappuoli R., Pizza M. 1998. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 187: 1123–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsen H., Adarna B. C., Schulz D., Rappuoli R., Jonsdottir I. 2001. Characterization of the antibody response to pneumococcal glycoconjugates and the effect of heat-labile enterotoxin on IGg subclasses after intranasal immunization. J. Infect. Dis. 183: 1494–1500 [DOI] [PubMed] [Google Scholar]
- 25.Taylor P. R., Pickering M. C., Kosco-Vilbois M. H., Walport M. J., Botto M., Gordon S., Martinez-Pomares L. 2002. The follicular dendritic cell restricted epitope, FDC-M2, is complement C4; localization of immune complexes in mouse tissues. Eur. J. Immunol. 32: 1888–1896 [DOI] [PubMed] [Google Scholar]
- 26.Kranich J., Krautler N. J., Heinen E., Polymenidou M., Bridel C., Schildknecht A., Huber C., Kosco-Vilbois M. H., Zinkernagel R., Miele G., Aguzzi A. 2008. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J. Exp. Med. 205: 1293–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngo V. N., Korner H., Gunn M. D., Schmidt K. N., Riminton D. S., Cooper M. D., Browning J. L., Sedgwick J. D., Cyster J. G. 1999. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189: 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraal G., Janse M., Claassen E. 1988. Marginal metallophilic macrophages in the mouse spleen: effects of neonatal injections of MOMA-1 antibody on the humoral immune response. Immunol. Lett. 17: 139–144 [DOI] [PubMed] [Google Scholar]
- 29.Phan T. G., Green J. A., Gray E. E., Xu Y., Cyster J. G. 2009. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 10: 786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer M. B., Ma M., Hsu N. C., Carroll M. C. 1998. Local synthesis of C3 within the splenic lymphoid compartment can reconstitute the impaired immune response in C3-deficient mice. J. Immunol. 160: 2619–2625 [PubMed] [Google Scholar]
- 31.Griffioen A. W., Rijkers G. T., Janssens-Korpela P., Zegers B. J. 1991. Pneumococcal polysaccharides complexed with C3d bind to human B lymphocytes via complement receptor type 2. Infect. Immun. 59: 1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston R. B., Jr., Altenburger K. M., Atkinson A. W., Jr., Curry R. H. 1979. Complement in the newborn infant. Pediatrics 64(5 Pt 2 Suppl): 781–786 [PubMed] [Google Scholar]
- 33.Pihlgren M., Fulurija A., Villiers M. B., Tougne C., Lambert P. H., Villiers C. L., Siegrist C. A. 2004. Influence of complement C3 amount on IgG responses in early life: immunization with C3b-conjugated antigen increases murine neonatal antibody responses. Vaccine 23: 329–335 [DOI] [PubMed] [Google Scholar]
- 34.Pasparakis M., Alexopoulou L., Episkopou V., Kollias G. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184: 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goriely S., Vincart B., Stordeur P., Vekemans J., Willems F., Goldman M., De Wit D. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166: 2141–2146 [DOI] [PubMed] [Google Scholar]
- 36.Pihlgren M., Tougne C., Schallert N., Bozzotti P., Lambert P. H., Siegrist C. A. 2003. CpG-motifs enhance initial and sustained primary tetanus-specific antibody secreting cell responses in spleen and bone marrow, but are more effective in adult than in neonatal mice. Vaccine 21: 2492–2499 [DOI] [PubMed] [Google Scholar]
- 37.Peppoloni S., Ruggiero P., Contorni M., Morandi M., Pizza M., Rappuoli R., Podda A., Del Giudice G. 2003. Mutants of the Escherichia coli heat-labile enterotoxin as safe and strong adjuvants for intranasal delivery of vaccines. Expert Rev. Vaccines 2: 285–293 [DOI] [PubMed] [Google Scholar]
- 38.Stephenson I., Zambon M. C., Rudin A., Colegate A., Podda A., Bugarini R., Del Giudice G., Minutello A., Bonnington S., Holmgren J., et al. 2006. Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers. J. Virol. 80: 4962–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis D. J., Huo Z., Barnett S., Kromann I., Giemza R., Galiza E., Woodrow M., Thierry-Carstensen B., Andersen P., Novicki D., et al. 2009. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE 4: e6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balogh P., Aydar Y., Tew J. G., Szakal A. K. 2001. Ontogeny of the follicular dendritic cell phenotype and function in the postnatal murine spleen. Cell. Immunol. 214: 45–53 [DOI] [PubMed] [Google Scholar]
- 41.Mandik-Nayak L., Huang G., Sheehan K. C., Erikson J., Chaplin D. D. 2001. Signaling through TNF receptor p55 in TNF-alpha-deficient mice alters the CXCL13/CCL19/CCL21 ratio in the spleen and induces maturation and migration of anergic B cells into the B cell follicle. J. Immunol. 167: 1920–1928 [DOI] [PubMed] [Google Scholar]
- 42.Olafsdottir, T. A. 2011. Neonatal immune response to vaccination: novel adjuvants and antigens to prevent pneumococcal and influenza infections. Doctoral dissertation, University of Iceland, Reykjavik, Iceland. [Google Scholar]
- 43.Bode C., Zhao G., Steinhagen F., Kinjo T., Klinman D. M. 2011. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 10: 499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun C. M., Deriaud E., Leclerc C., Lo-Man R. 2005. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 22: 467–477 [DOI] [PubMed] [Google Scholar]
- 45.Mackay F., Browning J. L. 1998. Turning off follicular dendritic cells. Nature 395: 26–27 [DOI] [PubMed] [Google Scholar]
- 46.Tritto E., Muzzi A., Pesce I., Monaci E., Nuti S., Galli G., Wack A., Rappuoli R., Hussell T., De Gregorio E. 2007. The acquired immune response to the mucosal adjuvant LTK63 imprints the mouse lung with a protective signature. J. Immunol. 179: 5346–5357 [DOI] [PubMed] [Google Scholar]
- 47.Breukels M. A., Zandvoort A., Rijkers G. T., Lodewijk M. E., Klok P. A., Harms G., Timens W. 2005. Complement dependency of splenic localization of pneumococcal polysaccharide and conjugate vaccines. Scand. J. Immunol. 61: 322–328 [DOI] [PubMed] [Google Scholar]
- 48.Buiting A. M., De Rover Z., Kraal G., Van Rooijen N. 1996. Humoral immune responses against particulate bacterial antigens are dependent on marginal metallophilic macrophages in the spleen. Scand. J. Immunol. 43: 398–405 [DOI] [PubMed] [Google Scholar]
- 49.Szakal A. K., Holmes K. L., Tew J. G. 1983. Transport of immune complexes from the subcapsular sinus to lymph node follicles on the surface of nonphagocytic cells, including cells with dendritic morphology. J. Immunol. 131: 1714–1727 [PubMed] [Google Scholar]
- 50.Ngo V. N., Cornall R. J., Cyster J. G. 2001. Splenic T zone development is B cell dependent. J. Exp. Med. 194: 1649–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Junt T., Moseman E. A., Iannacone M., Massberg S., Lang P. A., Boes M., Fink K., Henrickson S. E., Shayakhmetov D. M., Di Paolo N. C., et al. 2007. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450: 110–114 [DOI] [PubMed] [Google Scholar]
- 52.Cassese G., Arce S., Hauser A. E., Lehnert K., Moewes B., Mostarac M., Muehlinghaus G., Szyska M., Radbruch A., Manz R. A. 2003. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171: 1684–1690 [DOI] [PubMed] [Google Scholar]
- 53.Bossen C., Cachero T. G., Tardivel A., Ingold K., Willen L., Dobles M., Scott M. L., Maquelin A., Belnoue E., Siegrist C. A., et al. 2008. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood 111: 1004–1012 [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K., Grigorova I., Phan T. G., Kelly L. M., Cyster J. G. 2009. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J. Exp. Med. 206: 1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.