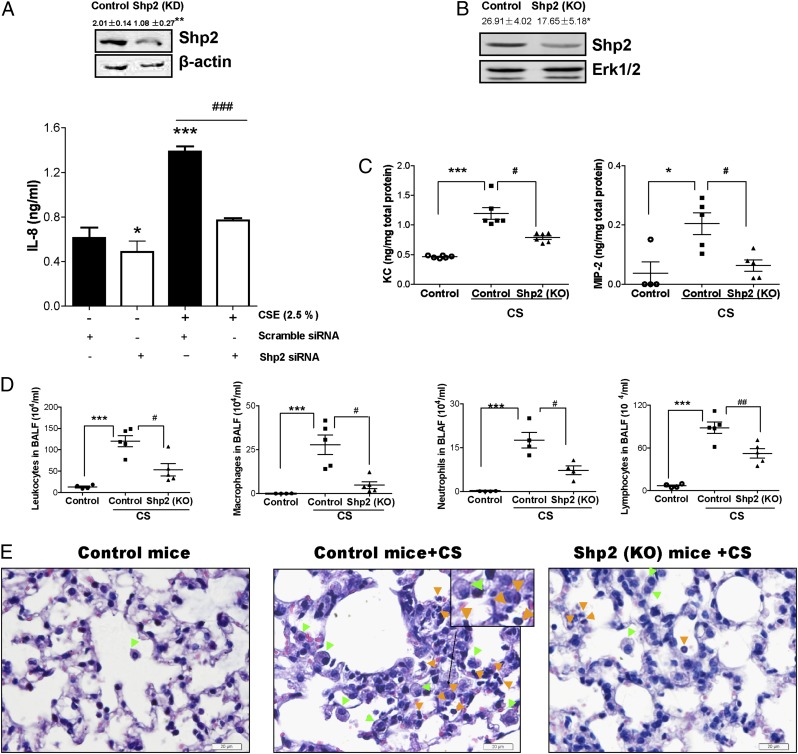
FIGURE 4.
Genetic ablation of Shp2 reduces the levels of IL-8 triggered by CSE in NCI-H292 cells and the inflammatory response induced by CS in mice. (A) The pulmonary epithelial cells were treated with transfection agent Lipofectamine LTX and transfected with either Shp2 siRNA (100 nM) or nonspecific control siRNA (100 nM). After 24 h, the cultured cells were treated with or without CSE (2.5%) for 24 h. The cells were collected to assess the levels of Shp2 production by immunoblotting assay, and the culture medium was used to measure IL-8 by ELISA. Data were expressed as mean ± SEM (n = 9 per group) of three independent experiments. *p < 0.05; **p < 0.01, ***p < 0.001 compared with cells (scramble siRNA) without CSE exposure. ###p < 0.001 compared with cells (Shp2 siRNA) with CSE exposure. (B) The Shp2 protein levels of Shp2 KO mice were evaluated by immunoblotting assays. Results were expressed as mean ± SEM of three independent experiments. *p < 0.05 compared with control mice. (C) Shp2 KO mice and control mice were repeatedly exposed to CS or laboratory air for 4 d. At 18 h after the last CS exposure, the BALFs were collected to evaluate the levels of KC and MIP-2 by ELISA. (D) The total leukocytes, macrophages, neutrophils, and lymphocytes were determined in BALFs. (E) Lung sections were stained with H&E, representative of three independent experiments. Mice with CS exposure, compared with air-exposed mice, showed macrophage (green arrowheads) and neutrophil (orange arrowheads) influx into the alveolar spaces. Shp2 KO mice exhibited a decrease in inflammatory cell infiltration. Results were expressed as mean ± SEM of three independent experiments. Scale bar, 20 μm. *p < 0.05, ***p < 0.001 compared with control mice with air exposure. #p < 0.05, ##p < 0.01 compared with control mice with CS exposure.