Abstract
Methodology for site-specific modification and chelate conjugation of a cyclic RGD (cRGD) peptide for the preparation of a radiotracer molecular imaging agent suitable for detecting αvβ3 integrin is described. The method involves functionalizing the peptide with an aldehyde moiety and conjugation to a 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) derivative that possesses an aldehyde reactive aminooxy group. The binding assay of the 111In-labeled peptide conjugate with αvβ3 integrin showed 60% bound when four equivalents of the integrin was added, a reasonable binding affinity for a mono-valent modified RGD peptide.
Keywords: Click Chemistry, aminooxy DOTA, RGD peptide, 203Pb
Introduction
Early detection of tumor cells is highly desirable and probably the most effective strategy towards the treatment and cure of cancer. It is well known that the progression and invasiveness of tumors in melanoma, glioma, and breast cancers correlates with αvβ3 integrin expression.1–5 Therefore, inhibition of αvβ3 integrin and thus suppression of tumor-induced angiogenesis, which leads to the termination of cancer metastasis, is important for the treatment of various carcinomas. In addition, targeting integrin and visualizing its expression non invasively using a radiotracer by positron emission tomography (PET) or single photon emission computed tomography (SPECT) imaging, would be highly advantageous for early detection of metastatic tumors.
The αvβ3 integrin is known to recognize the tripeptide sequence arginine-glycine-aspartic acid (RGD). It is also known that replacing any of the amino acids or changing the spatial conformation results in loss of binding activity, whereas cyclization of the peptide into an optimal conformation results in high affinity and selectivity.6–8 Over the course of several years, the use of radiolabeled cyclic RGD peptides as radiotracers for tumor imaging by SPECT or PET has been well documented.9–11 The cyclic RGD peptides have been employed as carrier vectors of metallic radionuclides, which are bound to the peptide through the use of bifunctional chelators, to target the αvβ3 integrin that was over-expressed on tumor cells for both imaging and therapy applications.6,12,13
The macrocyclic ligand 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) is employed in a variety of biomedical applications for sequestering metal ions relevant to medical applications. The complex formed with a lanthanide ion such as Gd3+ is clinically used as a diagnostic magnetic resonance imaging (MRI) agent, Dotarem.14 It can also be used as a bifunctional chelator (BFC) when attached to other functional molecules such as a protein or a peptide. It is well documented that DOTA is water soluble and forms highly stable complexes with many transition and rare earth metals (2+ and 3+ charged). This alleviates concerns related to the dissociation of radioactive metal ions or other toxicities, and thus leads to its popularity for in vivo sequestration of a variety of radionuclides and lanthanides alike.15–20 A number of DOTA derivatives have been extensively employed for a variety of metallic radionuclides such as 68Ga, 90Y, and 111In.21–23
Conjugation of peptides with DOTA derivatives has been mostly performed by solid phase synthesis using active ester analogs of the chelating agent generally as a tri-tert-butyl ester that lends itself to the convenience of this methodology and the associated deprotection protocols.24 This does however remove one of the carboxylate groups from the donor sphere about the metal ion or changes the character to an amide oxygen donor. In addition, there is the problem associated with the relatively long deprotection times necessary for the removal of the tert-butyl groups.25 Other researchers have reported classic solution based synthetic methods that utilized amino functionalities of peptide or proteins.23–29 A successful site-specific derivation of peptide or protein via the latter method can be tedious and often involves multi-step syntheses and protection of functional group(s) to avoid random conjugation that may compromise bioactivity of the derivatized compound. To obviate these limitations, click chemistry reactions that proceed efficiently and chemoselectively under physiological conditions were introduced recently as a more promising approach.30 Chemoselective click reactions between aminooxy derivatized DOTA and aldehyde31 or recombinant human serum albumin (rHSA)32 were previously reported. Herein, we extend this conjugation method to site-specifically conjugate the cyclic RGD peptide to aminooxy derivatized DOTA (AOD). The peptide was functionalized with an aldehyde moiety that is known to form a covalent oxime functional group bond with the aminooxy functional group that has been appended onto the DOTA ligand. In this fashion, the full eight coordinate symmetric sphere is preserved for optimal complex stability. The DOTA conjugate was then labeled with 111In and a binding assay was performed to determine the affinity of the peptide to the αvβ3 integrin to validate the conjugation and radiolabeling chemistry protocols as being suitable for such applications prior to performing biological experiments.
The radionuclide pair, 203Pb and 212Pb, possess favorable properties for use in diagnostic and therapeutic applications, respectively. 212 Lead (t1/2 = 10.6 h) serves as an ‘in vivo generator’ of 212Bi (t1/2 = 60 min) to overcome the short half-life of that daughter isotope.33 When DOTA is used to chelate 212Pb, the complex is adequately stable in vivo.34 Therefore, in addition to 111In, we explored the feasibility of labeling the RGD-DOTA conjugate with 203Pb (t1/2 = 52.0 h) which serves not only as a surrogate tracer for 212Pb, but also has potential for use as a SPECT imaging radionuclide (Eγ = 279.2 keV).34
Experimental
Materials and Methods
Phosphate buffered saline (PBS) at pH 7.4 was obtained from Digene (Gaithersburg, MD). The Reverse-phase HPLC (RP-HPLC) (method 1) was performed using a Beckman System Gold® (Fullerton, CA) equipped with a Model 126 solvent delivery module and a Model 168 UV-Vis detector with peak detection at 254 and 280 nm, and a C18 Ultrasphere™ column (250 × 10 mm; 5 µm). The flow rate was 2.5 mL/min and the mobile phase was isocratic with 90% A (0.1% TFA in water) and 10% B (0.1% TFA in acetonitrile) at 0 – 5 min, followed by a gradient mobile phase going from 10% B at 5 min to 90% B at 10 min. The mobile phase was then isocratic with 90% B at 10 – 18 min. All water used was purified using a Hydro Ultrapure Water Purification system (Rockville, MD). The cyclic RGD peptide was obtained from Peptide International (Louisville, Kentucky). The radiolabeled compounds were analyzed by RP-HPLC (method 2) on a Waters Breeze System consisting of a Binary HPLC Pump model 1525, an Autosampler model 717plus, monitored on line by a Dual Absorbance Detector model 2487 and a Beckman 170 Radioisotope Detector using a C-18 column (Beckman Coulter Ultrasphere 5 µm, 4.6×250 mm) and a linear gradient running from 100 % solvent A (0.1 M NH4OAc) to 100 % Solvent B (MeCN) in 30 min.
Succinimidyl-4-formylbenzoate (1)
The title compound was prepared as reported.35
2-(4-(3-(aminooxy)-2-oxopropyl)benzyl)1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (AOD) (2)
1-(2- cyclo (Arg-Gly-Asp-d-Phe-Lys)amido)4-formylbenzoate (cRGDf) (3)
Peptide (10 mg; 14.5 µmol), compound 1 (3.6 mg; 14.5 µmol), triethylamine (16.2g; 16.0 µmol) in DMF (400 µL) were stirred at room temperature for 38 h. The title compound was purified by RP-HPLC (method 1, tR: 16.4 min). ESI m/e: 823 (M+1).
cRGD-AOD (4)
Compound 3 (10 mg; 12.2 µmol) and Compound 2 (8.5 mg; 14.6 µmol) were stirred at room temperature in 0.1M sodium phosphate (0.5 mL; pH 4.5) for 6 h. The title compound was purified by RP-HPLC (method 1, tR: 4.8 min; 75% yield). ESI m/e: 696 (M+2).
Radiolabeling of 4 (111In-4 and 203Pb-4)
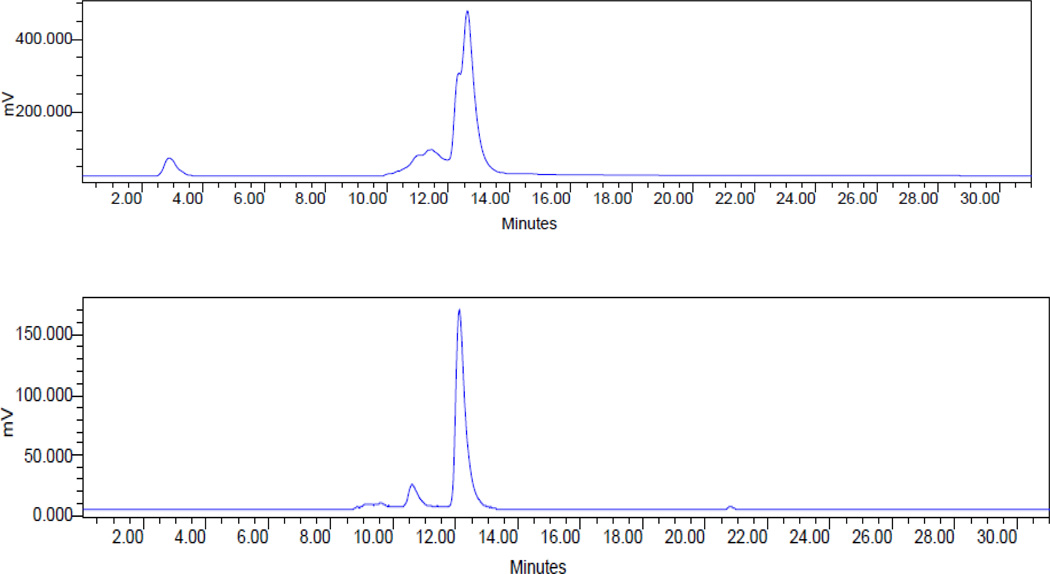
To a solution of 0.15 M NH4OAc (100 µL, pH = 7.0) was added 50 µL (1 mg/mL) of cRGDf-AOD and 2 µL of 111InCl3 solution (~500 µCi) in 0.05 N HCl. The reaction mixture was then incubated at 75 °C for 30 min. After heating, the vial was allowed to stand at room temperature for ~5 min. For 203Pb-4, radiolabeling could be completed in low pH (pH = 5.5) and by allowing the reaction mixture to incubate at 75 °C for 60 min. the resulting solution was analyzed and purified by HPLC (method 2, Figure 2)
Figure 2.
Radio-HPLC profiles of 111In-4 (top) and 203Pb-4 (bottom)
Binding assay with integrin αvβ3 receptor
The purified 111In-4 (2 × 106 cpm, 0.3 µM) was incubated with 0, 0.3, 0.6 and 1.2 µM of purified human αvβ3 integrin (Millipore, Temecula, CA) in a total volume of 25 µL PBS for 3 h at 37°C. For non-specific binding, excess 4 (to a final concentration of 20 µM) was added to reaction mixture. The reaction mixture was then separated on a 10 mL Sephadex G50 (Sigma, St. Louis, MO) column using PBS as eluent. Fractions (0.5 mL) were collected and counted on a γ-counter (Wallac, 1480 Wizard 3, Waltham, MA). The percent of the radioactivity bound to the receptor was then calculated (Table 1).
Table 1.
Binding of 111In-4 (0.3 µM) to a purified αvβ3 integrin.
| % Bound | |
|---|---|
| 111In-4 | 2.4 |
| 111In-4 + 0.3 µM Integrin | 10.5 |
| 111In-4 + 0.6 µM Integrin | 35.7 |
| 111In-4 + 1.2 µM Integrin | 60.5 |
| 111In-4 + 1.2 µM Integrin + 4 | 2.9 |
Result and Discussion
We chose to modify the peptide with an aldehyde rather than a ketone because aldehydes show much higher reactivity toward aminooxy groups or hydroxylamines than ketones.31 The reaction conditions reported here were optimal for modifying the peptide with an aldehyde group. The product formed was a 1:1 conjugate of the DOTA at the primary amine of the lysine residue, which is the only reactive amino site available under these conditions. In a previous study, the cyclic RGD peptide was functionalized with a hydroxylamine moiety and reacted with an 18F-labeled aldehyde to obtain a radiotracer that was used to study the αvβ3 integrin binding of the peptide.6,37,38 Here, we reversed the process by functionalizing the peptide with the aldehyde functionality in a single step and then reacted that product with a DOTA-hydroxylamine derivative to produce the ligand conjugate used to generate the 111In- and 203Pb-labeled radiotracer. The results showed that this synthetic route is quite convenient and efficient with moderate yields. Using the bifunctional DOTA ligand provides for the possibility of radiolabeling with a wide range of radionuclides, which is highly advantageous with a single precursor being suitable for multiple applications by just varying the radiolabel. Additionally, it is equally clear that the analogous chemistry would also support the incorporation and use of other bifunctional chelating agents and thus open up the range of possible radionuclides that might be attractive for targeting the αvβ3 integrin receptor.
The peptide conjugate was successfully radiolabeled with 111In and 203Pb (85% yield, Figure 2), while the reactive binding fraction of the resulting radiolabeled 111In product was measured as 35.7 and 60.5% when incubated with 0.6 and 1.2 µM of integrin. The receptor binding was completely blocked by 20 µM (~7-fold excess) of cold peptide conjugate (Table 1). Thus, we demonstrate that the radiolabeled products of this strategy provide products comparable to those prepared by other means.30–32,39
Conclusions
In summary, a new peptide-bifunctional chelate conjugate was synthesized with a moderate to high yield through a “click” oxime formation strategy. The bifunctional chelate was successfully radiolabeled with 111In and 203Pb, affording radiolabeled products suitable for SPECT imaging or γ-scintigraphy with reasonable reactivity and binding toward the target αvβ3 integrin. These results indicate that it is potentially possible to label this peptide conjugate with a large variety of radionuclides, the resulting radiotracer being suitable for in vivo tumor targeting. Further studies, such as biodistribution, in vivo tumor targeting and imaging, along with labeling with other radionuclides, are underway and will be reported in due course. We conclude that preservation of the full chelating potential of the DOTA chelating agent will lead to well defined stable products which, combined with the target binding ability of the peptide, will also make this conjugate attractive for potentially generating radiotherapeutic agents.
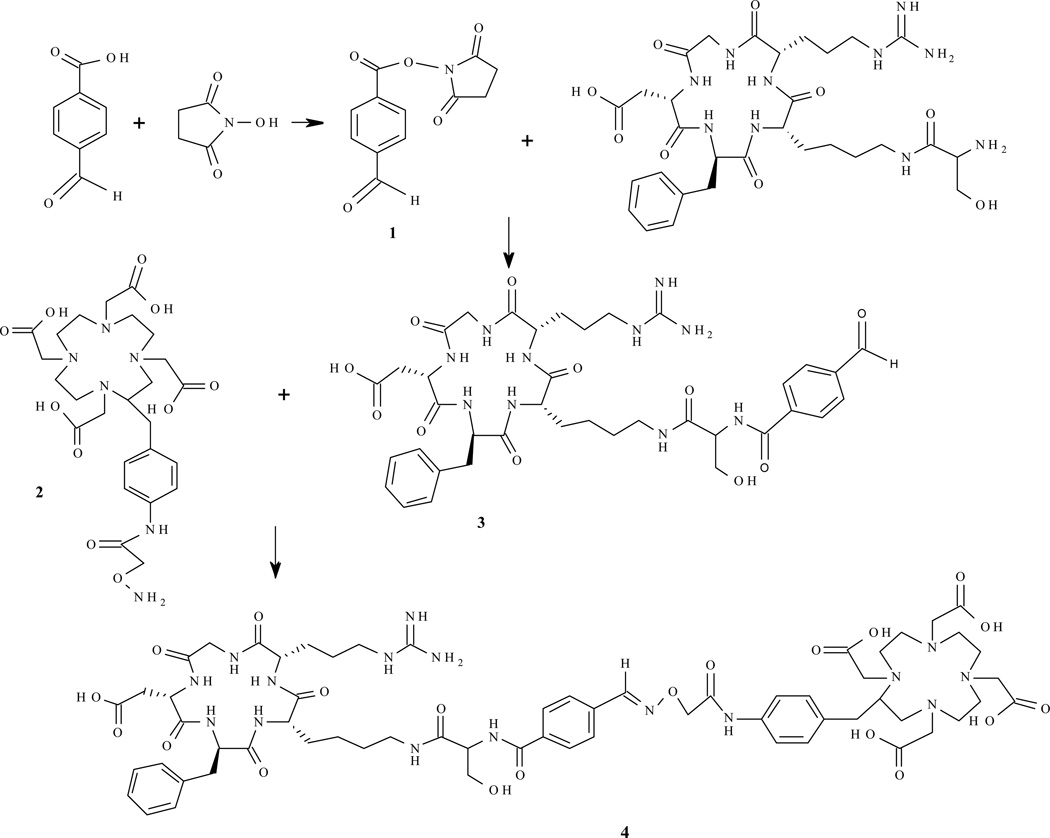
Figure 1.
Reaction scheme for preparation of the peptide conjugate.
Acknowledgment
This research was supported by the Intramural Research Program of the National Institute of Health, National Cancer Institute, Center for Cancer Research and the United States Department of Health and Human Services.
References
- 1.Bello L, Francolini M, Marthyn P, Zhang J, Carroll R, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black P. Neurosurgery. 2001;1:380. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Gasparini G, Brooks PC, Biganzoli E, Vermeulen PB, Bonoldi E, Dirix LY, Ranieri G, Miceli R, Cheresh DA. Clin. Cancer Res. 1998;4:2625. [PubMed] [Google Scholar]
- 3.Albelda SM, Damjanovich L, Herlyn M, Buck CA. Cancer Res. 1990;50:6757. [PubMed] [Google Scholar]
- 4.Zitzmann S, Ehemann V, Schwab M. Cancer Res. 2002;62:5139. [PubMed] [Google Scholar]
- 5.Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA. J. Clin. Invest. 1992;89:2018. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haubner R, Finsinger D, Kessler H. Angew. Chem. Int. Ed. Engl. 1997;36:1374. [Google Scholar]
- 7.Gurrath M, MÜller G, Kessler H, Aumailley M, Timpl R. E. J. Biochem. 1992;210:911. doi: 10.1111/j.1432-1033.1992.tb17495.x. [DOI] [PubMed] [Google Scholar]
- 8.Aumailley M, Gurrath M, Müller G, Calvete J, Timpl R, Kessler H. FEBS Lett. 1991;291:50. doi: 10.1016/0014-5793(91)81101-d. [DOI] [PubMed] [Google Scholar]
- 9.Liu S. Bioconj. Chem. 2009;20:2199. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Bioconj. Chem. 2009;20:750. doi: 10.1021/bc800455p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J, Kim YS, Chakraborty S, Jia B, Wang F, Liu S. Bioconj. Chem. 2009;20:1559. doi: 10.1021/bc9001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Edwards DS. Chem. Rev. 1999;99:2235. doi: 10.1021/cr980436l. [DOI] [PubMed] [Google Scholar]
- 13.Liu S. Chem. Soc. Rev. 2004;33:445. doi: 10.1039/b309961j. [DOI] [PubMed] [Google Scholar]
- 14.Lauffer RB. Chem. Rev. 1987;87:901. [Google Scholar]
- 15.Corneillie TM, Whetstone PA, Fisher AJ, Meares VF. J. Am. Chem. Soc. 2003;124:3436. doi: 10.1021/ja029363k. [DOI] [PubMed] [Google Scholar]
- 16.Loncin MF, Desreux JF, Merciny E. Inorg. Chem. 1986;25:2646. [Google Scholar]
- 17.Moi MK, Meares CF, DeNardo SJ. J. Am. Chem. Soc. 1988;110:6266. doi: 10.1021/ja00226a063. [DOI] [PubMed] [Google Scholar]
- 18.Wu AM, Yazaki PJ, Tsai SW, Nguyen K, Anderson AL, McCarthy DW, Welch MJ, Shively JE, Williams LE, Raubitschek AA, C. Wong JY, Toyokuni T, Phelps ME, Gambhir SS. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8495. doi: 10.1073/pnas.150228297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, Haldemann A, Mueller-Brand J. J. Nucl. Med. 2002;43:610. [PubMed] [Google Scholar]
- 20.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Chem. Rev. 2010;110:2858. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Leon-Rodriquez LM, Kovacs Z. Bioconj. Chem. 2008;19:391. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 22.Ansquer C, Kraeber-Bodere F, Chatal JF. Curr. Pharm. Design. 2009;15:2453. doi: 10.2174/138161209788682262. [DOI] [PubMed] [Google Scholar]
- 23.P. Breeman WA, de Blois E, Chan HS, Konijnenberg M, Kwekkeboom DJ, Krenning EP. Semin. Nucl. Med. 2011;41:314. doi: 10.1053/j.semnuclmed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Heppeler A, Froidevaux S, Mäcke HR, Jermann E, Béhé M, Powell P, Hennig M. Chem. Eur. J. 1999;5:1974. [Google Scholar]
- 25.Wängler B, Beck C, Wanger-Utermann U, Schirrmacher E, Bauer C, Rösch F, Schirrmacher R, Eisenhut M. Tetrahedron Lett. 2006;47:5985. [Google Scholar]
- 26.Mier W, Hoffend J, Krämer S, Schuhmacher J, Hul WE, Eisenhut M, Haberkorn U. Bioconj. Chem. 2004;16:237. doi: 10.1021/bc034216c. [DOI] [PubMed] [Google Scholar]
- 27.Martin S, O’Donnell R, Kukis D, Abbey C, McKnight H, Sutcliffe J, Tuscano J. Mol. Imaging Biol. 2009;11:79. doi: 10.1007/s11307-008-0148-1. [DOI] [PubMed] [Google Scholar]
- 28.Schlesinger J, Koezle I, Bergmann R, Tamburini S, Bolzati C, Tisato F, Noll B, Klussmann S, Vonhoff S, Wuest F, Pietzsch HJ, Steinbach J. Bioconj. Chem. 2008;19:928. doi: 10.1021/bc700453h. [DOI] [PubMed] [Google Scholar]
- 29.Wängler C, Wängler B, Eisenhut M, Haberkorn U, Mier W. Bioorg. Med. Chem. 2008;16:2606. doi: 10.1016/j.bmc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 30.Wangler C, Schirrmacher R, Bartenstein P, Wangler B. Curr. Med. Chem. 2011;17:1092. doi: 10.2174/092986710790820615. [DOI] [PubMed] [Google Scholar]
- 31.Wängler C, Schäfer M, Schirrmacher R, Bartenstein P, Wängler BB. Bioorg. Med. Chem. 2011;19:3864. doi: 10.1016/j.bmc.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Young NL, Whetstone PA, Cheal SM, Benner WH, Lebrilla CB, Meares CF. J. Proteome. Res. 2006;5:539. doi: 10.1021/pr050299q. [DOI] [PubMed] [Google Scholar]
- 33.Yong K, Brechbiel MW. Dalton Trans. 2011;40:6068. doi: 10.1039/c0dt01387k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milenic DE, Roselli M, Brechbiel MW, Pippin CG, McMurry TJ, Carrasquillo JA, Colcher D, Lambrecht R, Gansaw OA, Schlom J. Eur. J. Nucl. Med. 1998;25:471. doi: 10.1007/s002590050246. [DOI] [PubMed] [Google Scholar]
- 35.Phillips JA, Morgan EL, Dong Y, Cole GT, McMahan C, Hung CY, Sanderson SD. Bioconj. Chem. 2009;20:1950. doi: 10.1021/bc9002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takenouchi K, Tabe M, Watanabe K, Hazato A, Kato Y, Shionoya M, Koike T, Kimura E. J. Org. Chem. 1993;58:6895. [Google Scholar]
- 37.Glaser M, Morrison M, Solbakken M, Arukwe J, Karlsen H, Wiggen U, Champion S, Kindberg GM, Cuthbertson A. Bioconj. Chem. 2008;19:951. doi: 10.1021/bc700472w. [DOI] [PubMed] [Google Scholar]
- 38.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M. Cancer Res. 2001;61:1781. [PubMed] [Google Scholar]
- 39.Knor S, Modlinger A, Poethko T, Schottelius M, Wester HJ, Kessler H. Chem. Eur. J. 2007;13:6082. doi: 10.1002/chem.200700231. [DOI] [PubMed] [Google Scholar]