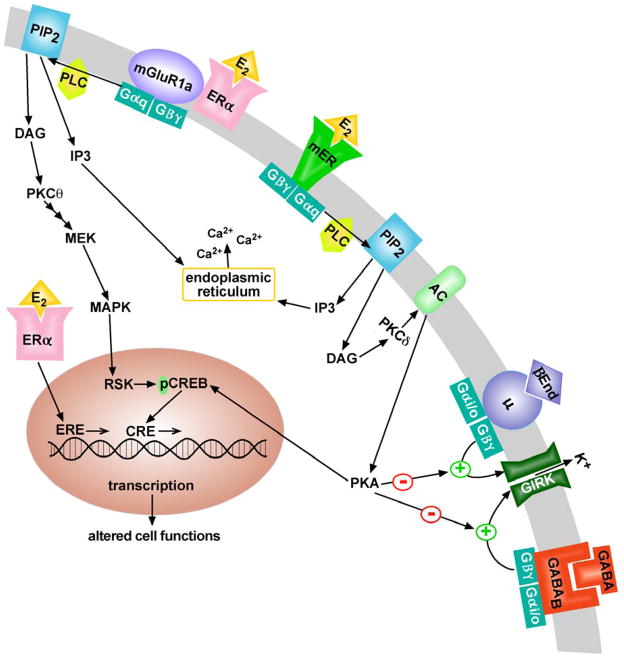
Figure 1.
Proposed schema of E2 signaling in POMC neurons: As in other non-neural cells, E2 binds to intracellular ERα to activate genes via estrogen-response elements (EREs). In addition, E2 binds to ERα localized at the neuronal (plasma) membrane and activates group 1 metabotropic glutamate receptor (mGluR1a) signaling. Activation of mGluR1a causes Gαq stimulation of phospholipase C (PLC), which leads to the hydrolysis of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). IP3 releases internal calcium (Ca2+) stores, while DAG activates protein kinase C-theta (PKCθ) leading to mitogen-activated kinase kinase (MEK) to mitogen-activated kinase (MAPK) to ribosomal S6 kinase (RSK)-induced phosphorylation of cAMP response element-binding protein (CREB). pCREB will activate genes expressing CREB-response elements (CREs). Also, E2 activates a membrane-associated ER (mER) that is Gαq -coupled (Gq-mER) to activate PLC generating IP3 and DAG. Ca2+ is released from the endoplasmic reticulum by IP3, and DAG activates protein kinase C-delta (PKCδ). Through phosphorylation, adenylyl cyclase (AC) activity is upregulated by PKCδ. The generation of cAMP activates PKA, which through phosphorylation can rapidly uncouple GABAB and μ-opioid (μ) receptors from G protein-coupled, inwardly rectifying K+ (GIRK) channels. Gq-mER-mediated activation of PKA can also phosphorylate CREB. Therefore in addition to the direct effects on membrane excitability, both mGluR1 and mER signaling pathways can alter gene transcription through CREB response elements (CREs) on genes.