Abstract
To describe the spectrum and associated clinical features of peripheral and cerebral vasculopathy in pediatric patients with neurofibromatosis type 1, children seen at a single center from 2000–2010 with appropriate imaging studies were identified. Scans were assessed for vascular disease by two pediatric neuroradiologists. Of 181 children, 80 had pertinent imaging studies: 77 had brain imaging, 6 had peripheral imaging, and 3 had both. Vasculopathy was identified in 14/80 children (18%, minimum prevalence of 14/181; 8%). Of those with vascular abnormalities, 2/14 had peripheral vasculopathy (1% minimum prevalence) and 12/14 had cerebrovascular abnormalities (7% minimum prevalence). No associations were found between vasculopathy and common clinical features of neurofibromatosis type 1 including optic pathway glioma, plexiform neurofibroma, skeletal abnormalities, attention deficit hyperactivity disorder, or suspected learning disability. Both peripheral and cerebral vasculopathy are important complications of pediatric neurofibromatosis type 1 and should be considered in the management of this complex disease.
Keywords: Neurofibromatosis Type I, Phakomatosis, Children, Moyamoya, Peripheral Vasculopathy
INTRODUCTION
Neurofibromatosis type 1 is an autosomal dominant genetic disorder caused by mutation in the tumor suppressor gene NF1.1 The protein product of NF1 is neurofibromin, a guanosine triphosphatase activating protein that negatively regulates Ras activity.2,3 Neurofibromatosis type 1 is characterized by nerve sheath neurofibromas, but has a number of additional clinical features. One of these clinical features is vasculopathy, which can occur throughout the body (brain and periphery).4–7
The etiology of vascular disease in neurofibromatosis type 1 is not well understood. The neurofibromin protein has been shown to be expressed in the vascular endothelial cell layer as well as in the smooth muscle of the aorta, and is likely involved in the pathogenesis of neurofibromatosis type 1-associated vasculopathy.8,9 Smooth muscle cells that have lost NF1 exhibit an abnormal proliferative response to arterial injury, which may account for the development of obstructive vascular disease in neurofibromatosis type 1.3 This increased neointima formation in response to mechanical injury has been shown to be mediated by a molecular signaling pathway in NF1+/− mice that is sensitive to the tyrosine kinase inhibitor imatinib (Novartis).10
With this genetic predisposition, vasculopathy in neurofibromatosis type 1 appears to be an acquired condition. The progression of existing vascular lesions, as well as the development of new lesions, have been described in individuals with neurofibromatosis type 1.11,12 Yearly blood pressure monitoring is recommended for children with neurofibromatosis type 1 due to the possible development of hypertension associated with renovascular disease, coarctation of the aorta, or other peripheral vascular abnormalities.13,14 Screening imaging studies, such as magnetic resonance imaging and magnetic resonance angiography of the brain or renovascular imaging, in children without symptoms are not recommended.13 A better understanding of vascular disease in pediatric neurofibromatosis type 1 would inform the clinical management of these children.
Several studies have examined the prevalence and spectrum of cerebrovascular disease in children with neurofibromatosis type 1.4–6 These authors have found that moyamoya syndrome is fairly common, present in 3–5% of children with neurofibromatosis type 1 with imaging performed for clinical indications5,6 and representing 60–70% of children with neurofibromatosis type 1 and cerebrovascular abnormalities.6 In these studies, children with milder forms of cerebrovascular disease were often asymptomatic.4–6 Other types of neurofibromatosis type 1-associated vascular disease including aortic and renal artery abnormalities have been discussed in only small case series.15,16
Various abnormalities have been reported in vessels of all calibers, but neither the frequency of non-cerebral vascular disease in neurofibromatosis type 1 patients nor the association between cerebral and peripheral vasculopathy has been systematically studied.7 The aim of our study was to describe the spectrum of both peripheral and cerebral vasculopathy in a tertiary care clinical population of pediatric neurofibromatosis type 1 patients, to estimate the minimum prevalence of vasculopathy in this population, and to examine associations between vasculopathy and common features of neurofibromatosis type 1.
METHODS
Study Population
The Comprehensive Neurofibromatosis Center at the Johns Hopkins Hospital provides care for children and adults with confirmed neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis. In keeping with the current recommendations from the National Neurofibromatosis Foundation Optic Pathway Task Force17 and the American Academy of Pediatrics,13 screening or universal imaging is not performed; instead, imaging is requested for clinical concerns. The study population was drawn from the Institutional Review Board-approved Comprehensive Neurofibromatosis Center database that included adults and children seen from July 2000 through June 2010. Inclusion criteria were 1) children 0–18 years during the study period who met National Institutes of Health diagnostic criteria for neurofibromatosis type 1; 2) with appropriate imaging (defined below) during the study period. Patients who received intracranial radiation were not excluded. This study was approved by the Institutional Review Board.
In order to examine associations between vasculopathy and common features of neurofibromatosis type 1, clinical and demographic information for each study patient was obtained from the electronic patient record. For bony deformities, sphenoid wing dysplasia, tibial bowing, pseudoarthrosis were all included; moderate to severe scoliosis or kyphosis was also reported, defined as a curvature of at least 25°.18,19 The presence of physician-diagnosed attention deficit hyperactivity disorder and/or suspected learning disability (defined as the child having repeated at least one grade, having an individual education plan, or attending a special education school) was also recorded. Hypertension was defined as diagnosed by a physician; recorded blood pressures were compared to national guidelines for height, weight, and gender.20
Imaging
Patients with existing imaging studies completed between 2000 and 2010 were identified. The following imaging studies were used to examine the peripheral vasculature: magnetic resonance angiography of the abdomen and chest, contrast-enhanced computed tomography, computed tomography-angiography or non-cerebral conventional angiography of the abdomen and chest. The following imaging studies were examined for vasculopathy of the head and neck: magnetic resonance imaging of the brain, magnetic resonance angiography of the brain and/or neck, conventional cerebral angiography, and computed tomography of the neck. Dedicated imaging of the spine was not used for the evaluation of the neck, thoracic or abdominal vasculature due to the limited and inconsistent ability of these scans to visualize the vasculature. The first and most recent available imaging study of each modality were reviewed to examine the progression of vascular findings over time.
All imaging studies were reviewed independently by two pediatric radiologists who are also board-certified in neuroradiology; they were blinded to the clinical presentation.6 Vasculopathy was defined as any abnormality in the cerebral or peripheral vasculature that could not be considered a normal variant. In particular, vessels were examined for stenosis, aneurysm, tortuosity, and the formation of abnormal collateral arterial supply. Appearance of moyamoya vessels, prior ischemic injury, and prior revascularization surgery was noted. The presence of optic pathway glioma, other glioma, plexiform neurofibroma, sphenoid wing dysplasia, and focal areas of abnormal signal intensity (also referred to as unidentified bright objects in neurofibromatosis type 1) was also noted. In cases of disagreement, consensus opinion based on discussion between the two radiologists was sought and reported.
Statistical Analysis
Frequency of vasculopathy was described. We compared the frequency of cerebral vasculopathy in our study population to those of Rosser, Cairns, and Rea4–6 via a binomial comparison of proportions. All comparisons of proportions were analyzed using chi square or Fisher’s exact test when any value was less than five. Due to the small number of outcome events, logistic regression was not performed but rather Fisher’s exact test was used to examine features associated with cerebral vasculopathy, peripheral vasculopathy and any vasculopathy. We conducted analyses using STATA 11.0 (College Station, TX) and considered a p-value of <0.05 to be significant for all analyses.
RESULTS
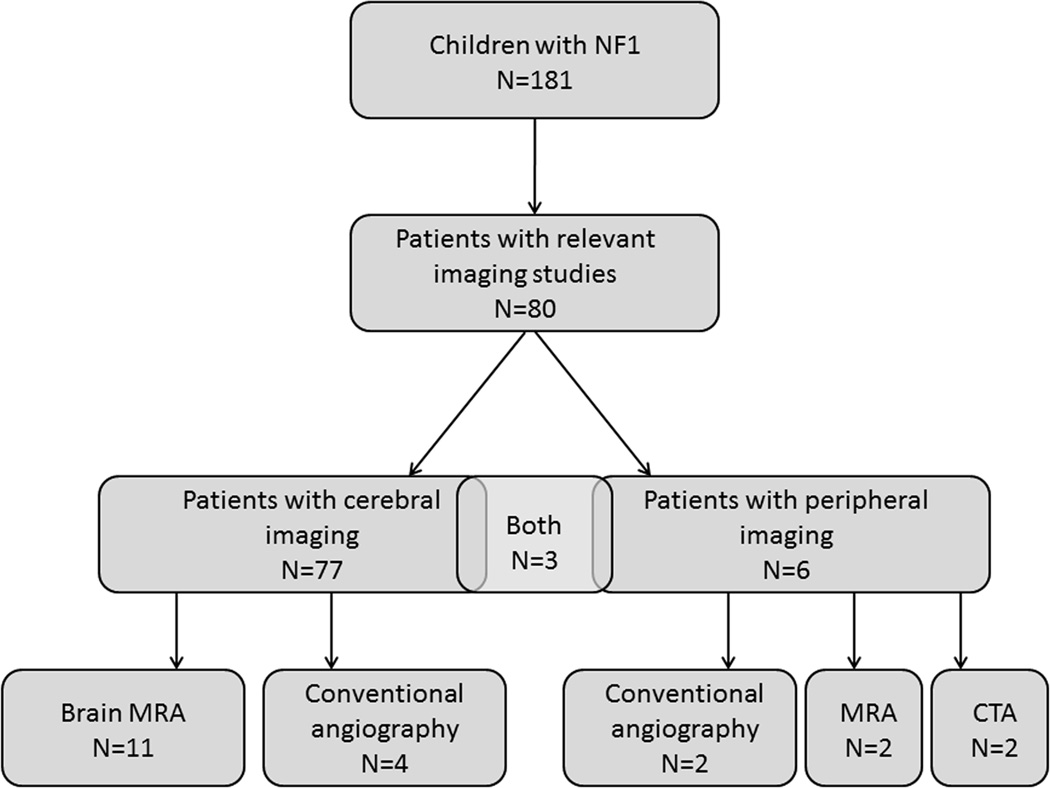
We screened 517 patients in the neurofibromatosis database and found 424 adults and children with a confirmed diagnosis of neurofibromatosis type 1. Of these, 181 were between 0–18 years of age. Of this group of 181 children, 80 (44%) had pertinent and available imaging studies completed between 2000 and 2010. Demographic information is summarized in Table 1. Indications for imaging are tabulated in Table 2, and clinical characteristics of patients with identified vasculopathy are summarized in Table 3. All imaging studies were performed for clinical indications except eight brain magnetic resonance images ordered for screening purposes by outside physicians prior to referral to our neurofibromatosis center. Of the magnetic resonance imaging studies examined, 95% were performed using a 1.5-tesla scanner and 5% on a 3-tesla scanner. Of 80 children with imaging (Figure 1, Flow Chart), 77 (96%) had magnetic resonance images of the brain and 11 patients had cerebral magnetic resonance angiography. Four children had conventional angiography of the cerebral vasculature to better define severe vasculopathy. Of the six children with peripheral imaging, two had magnetic resonance angiography of the abdomen/pelvis, two had computed tomography angiography, and two had conventional angiography of the abdominal aorta or its major branches. Three patients had both central and peripheral imaging studies.
Table 1.
Demographics and associated diagnoses of pediatric neurofibromatosis type 1 study population (n=80)
| Parameter | Our findings (n) |
|---|---|
| Gender | 40% female (32) 60% male (48) |
| Age | 12.2 ± 0.4 years |
| Family history of NF1 | 49% (39) |
| Learning Disabilities | 53% (42) |
| ADHD | 29% (23) |
| Plexiform neurofibroma | 36% (29) |
| Optic pathway glioma* | 34% (26) |
| Other glioma* | 10% (8) |
| FASI* | 79% (61) |
| Bony deformities | 19% (15) |
| Scoliosis/kyphosis | 16% (13) |
| Hypertension | 6% (5) |
Abbreviations: ADHD, attention deficit hyperactivity disorder; FASI, focal areas of abnormal signal intensity; NF1, neurofibromatosis type 1.
N=77 for these variables.
Table 2.
Indications for Imaging in the Study Population (n=80)
| Indication for Imaging | Number of Patients* |
|---|---|
| Central Nervous System Imaging | |
| OPG/visual changes | 23 |
| Head/neck mass | 8 |
| Stroke-like symptoms (e.g. weakness, cranial nerve palsy, gait disturbance) | 8 |
| Screening† | 8 |
| Headache | 7 |
| Seizure | 7 |
| Other/Unknown | 16 |
| Peripheral Imaging | |
| Hypertension | 4 |
| MPNST | 1 |
| Low back pain | 1 |
Abbreviations: OPG, optic pathway glioma; MPNST, malignant peripheral nerve sheath tumor.
Patients may be listed more than once if they had more than one indication for imaging.
Images obtained for screening purposes were ordered by outside clinics.
Table 3.
Characteristics of Children with Neurofibromatosis Type 1-Associated Vasculopathy (N=14)
| Patient Number |
Sex | Age at Diagnosis of Vasculopathy (y) |
Indications for Imaging | Symptoms of Vasculopathy | Vascular Findings on Imaging | Treatment | Evidence of Progression |
Follow-up (y) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 5 | Seizure, stroke-like symptoms | Stroke (Right-sided weakness, decreased responsiveness) | Moyamoya; left ACA, MCA, ICA stenosis; left hemisphere stroke | Surgery, aspirin | No | 6.5 |
| 2 | F | 9 | Right-sided weakness | TIA (hemiparesis) at age 3, clinically stable for 6 years | Moyamoya; right MCA/ICA stenosis | Aspirin | No | <1 |
| 3 | F | 4 | OPG | None | Moyamoya; bilateral ACA, MCA, ICA stenosis | None | No | 8 |
| 4 | M | 3 | OPG | None | Moyamoya; bilateral ACA, MCA, left PCA stenosis; left subcortical stroke | Surgery, aspirin | No | 1.2 |
| 5 | M | 9 | OPG | None | Moyamoya; right MCA stenosis | None | Yes | 7.4 |
| 6 | M | 12 | Chiari -1 malformation | None | Left ICA occlusion, DVA | None | No | <1 |
| 7 | M | 8 | Neck mass, ganglioglioma | None | Right ICA/VA tortuosity, DVA | Surgery | No | <1 |
| 8 | M | 12 | Headaches | None | Bilateral MCA/ICA elongation | None | N/A* | 0 |
| 9 | F | 13 | Neck mass, PN | None | Cavernous ICA tortuosity | None | No | 1.8 |
| 10 | M | 6 | Facial neurofibroma | None | Left ICA stenosis, displacement | None | N/A* | 0 |
| 11 | M | 8 | Mood changes, wide-based gait | None | Bilateral MCA/ICA elongation | None | No | 1.5 |
| 12 | M | 11 | OPG, unusual gait | None | Bilateral ICA tortuosity | None | Yes | 7 |
| 13 | F | 7 | Hypertension | Hypertension, seizures, renal failure | Mid aortic syndrome: stenosis of abdominal aorta, SMA, celiac trunk, RAs | Surgery, anti- hypertensive medication, aspirin | Yes | 3 |
| 14 | M | 5 | Hypertension | Hypertension | Mid aortic syndrome: suprarenal abdominal aortic stenosis | Surgery, anti-hypertensive medication | Yes†* | 0 |
Abbreviations: ACA, anterior cerebral artery; DVA, developmental venous anomaly; ICA, internal carotid artery; MCA, middle cerebral artery, PCA, posterior cerebral artery; PN, plexiform neurofibroma; OPG, optic pathway glioma; RA, renal artery; SMA, superior mesenteric artery; TIA, transient ischemic attack; VA, vertebral artery.
Indicates a patient without an available follow-up study
Follow-up images were not available for review, but the child progressed clinically with difficult-to-control hypertension and progression of aortic vasculopathy per outside radiology report. He had surgical revascularization for mid-aortic syndrome at an outside institution.
Figure 1.
Flow Diagram of Type of Imaging for Children with Neurofibromatosis Type I.
Abbreviations: MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; NF1, neurofibromatosis type 1
Vasculopathy was identified in 14/80 children (18%) who had imaging, for a minimum prevalence of 14/181 (8%) in the study population. Of 14 children with identified vascular abnormalities, 2 (14%) had abnormalities of the peripheral vasculature and 12 (86%) had cerebral vasculopathy (Figure 2). The minimum prevalence values based on a study population of 181 were 1% and 7% for peripheral and cerebral vasculopathy respectively. None of the three children with central and peripheral imaging had both cerebrovascular and peripheral vascular abnormalities. The mean duration of follow-up, defined as time elapsed between the initial and the most recent imaging study, was 6.3 years with a standard deviation of 4 years.
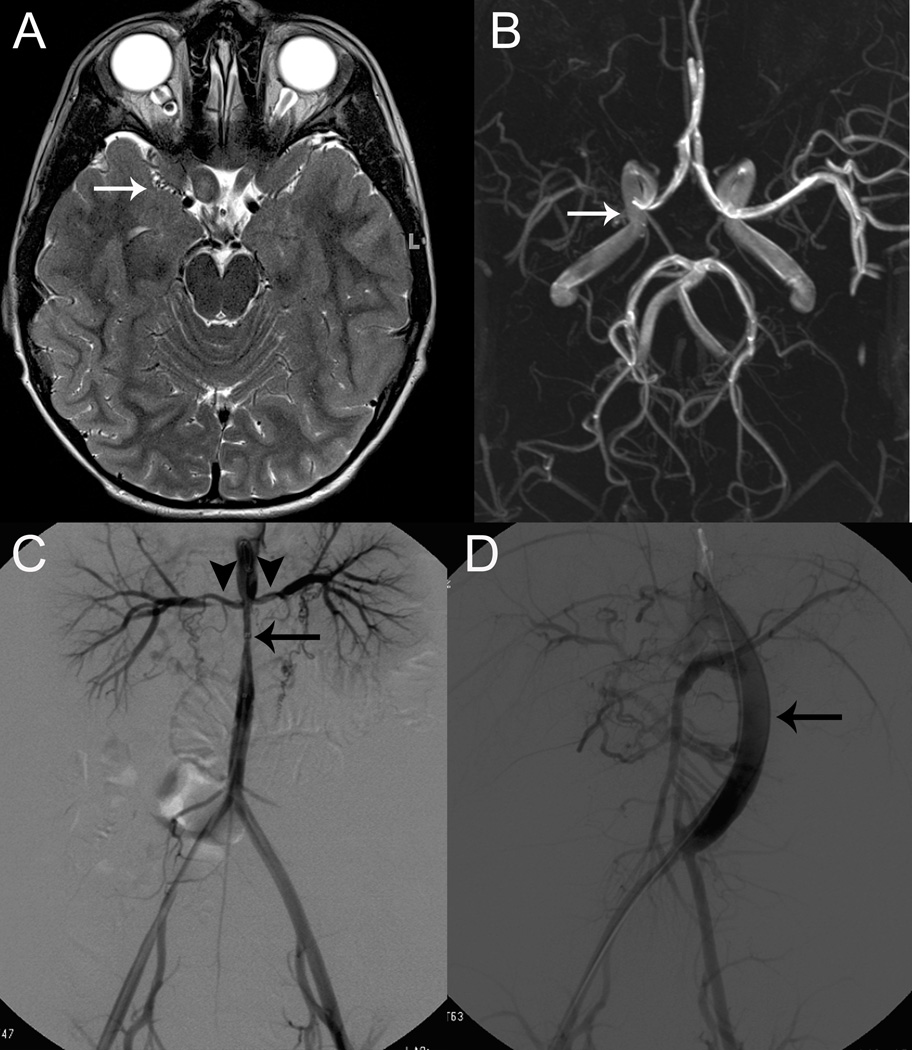
Figure 2.
Cerebral and Peripheral Vasculopathy in Two Children with Neurofibromatosis Type 1
A. Axial, T2-weighted magnetic resonance imaging for Patient #5 shows bilateral optic pathway gliomas (arrowheads) with evidence of moyamoya vessel flow voids along the expected course of the right middle cerebral artery (arrow).
B. Axial maximum intensity projection of a three-dimensional time-of-flight magnetic resonance angiography reveals complete occlusion of the proximal right middle cerebral artery, arrow. These findings were discovered incidentally on routine magnetic resonance imaging to follow optic pathway gliomas, and the patient has remained asymptomatic without treatment.
C. Digital subtraction angiography of the abdominal aorta of patient #13 following initial presentation with malignant hypertension and status epilepticus, reveals mid-aortic syndrome (arrow) with involvement of the renal arteries (arrowheads).
D. Renal arteriogram following surgical treatment of mid-aortic syndrome including aorto-aortic bypass and right renal artery and superior mesenteric artery bypass grafting. Angioplasty was later performed on the right renal artery due to persistent stenosis near its takeoff point from the aortic graft. A jump graft (arrow) connects the distal thoracic aorta to the abdominal aorta just above the bifurcation.
Peripheral Vasculopathy
Only 6 of 80 children had pertinent imaging of the peripheral vasculature; two of six children with appropriate imaging had peripheral vasculopathy and were diagnosed with mid-aortic syndrome, defined as stenosis of multiple branches of the abdominal aorta.21,22 Both of the children with peripheral vasculopathy met national guidelines for stage 2 hypertension: systolic and/or diastolic BP ≥99th percentile plus 5 mmHg for age, height and gender20 and had fairly dramatic hypertension at presentation with systolic blood pressures >160 mmHg. There were 3 of 5 children with hypertension who did not have a vascular explanation for their elevated blood pressure (one child only had a renal duplex ultrasound and brain magnetic resonance imaging; he met study inclusion criteria based on brain MRI but did not have appropriate peripheral vascular imaging studies). After undergoing evaluation for pheochromocytoma and other causes of hypertension, these patients were diagnosed with idiopathic hypertension and two of the three required medical management of their blood pressure. Both children with mid-aortic syndrome showed progression of their vasculopathy (Table 3) and required surgical treatment of their vasculopathy in addition to multiple antihypertensive medications for control of their blood pressure. Both children underwent magnetic resonance imaging of the brain and did not have cerebral vasculopathy.
Cerebral Vasculopathy
Cerebral vasculopathy was found in 12 of 77 children who underwent neuroimaging (15%, 95% CI:8%-26%). Moyamoya syndrome was identified in 5 of 77 (6%) children and was the most common vascular abnormality. Of the children with moyamoya, the most severe form of cerebral vasculopathy, two were symptomatic -- with clinical stroke in one child and transient ischemic attack in the other; three children were asymptomatic and were diagnosed with abnormal “moyamoya vessels” seen on magnetic resonance imaging of the brain done for another indication (concern for optic pathway glioma or headache). One clinically asymptomatic child with moyamoya had a silent stroke (no focal deficits, stroke not suspected) detected on imaging. Both children with overt or silent stroke seen on magnetic resonance imaging of the brain had cerebral vasculopathy. Two of the 12 patients with cerebral vasculopathy exhibited progression of their vasculopathy on subsequent imaging. One patient developed moyamoya that became apparent seven years after serial magnetic resonance images for optic pathway glioma were initiated and the other had tortuosity of the intracranial internal carotid artery that progressed over seven years. Two of five children underwent revascularization surgery for moyamoya syndrome. Of the 80 children with imaging, one had received cranial radiation therapy for a pontine glioma; this child was subsequently followed for six months without evidence of vasculopathy on magnetic resonance imaging.
Other Neurofibromatosis Type I Associated Diagnoses
Of the 77 children with magnetic resonance imaging of the brain, 34% had an optic pathway glioma, and other presumed glial brain tumors were seen in 10%; 79% had focal areas of abnormal signal intensity (also called unidentified bright objects). Of the 80 children with imaging who were included in the study population, plexiform neurofibromas were present in 29 children (36%). Bony deformities were found in 19% and moderate to severe scoliosis or kyphosis was found in 16%. Cognitive and behavior diagnoses were also common with 29% of patients having physician-diagnosed attention deficit hyperactivity disorder and 53% having a suspected learning disability.
Analysis of Associations with Vasculopathy
In this clinic-based population, we found no association with, or predictors of, vasculopathy including gender, focal areas of abnormal signal intensity, plexiform neurofibroma, or the presence of optic pathway glioma. In particular, attention deficit hyperactivity disorder and suspected learning disability were not associated with cerebral vasculopathy. Severe hypertension was present in both children with severe vasculopathy of the abdominal aorta. Hypertension was not associated with cerebral vasculopathy.
Comparison to Similar Studies
We did not find other studies that examined the prevalence of peripheral vasculopathy. When comparing these data to the three prior studies of cerebral vasculopathy, we report a prevalence that is 8–11% higher than the existing data. Cairns et al. found cerebral vasculopathy in 7 of 144 children (5%, 95% CI: 2–10%) with neurofibromatosis type 1 who underwent brain magnetic resonance imaging compared with 12 of 77 children (15%, 95% CI:8%-26%)) in this current study, a 10% difference in proportions that is significant (95% CI 0.9% to 18%, p=0.01). Rosser et al. noted cerebral vasculopathy in 8 of 316 children (2.5%, 95% CI: 2–5%) with neurofibromatosis type 1 who had magnetic resonance imaging of the brain; again, compared with the prevalence of vasculopathy in our study, there is an 11% difference in proportions which was significant (95% CI: 4–19%, p<0.0001). Finally, Rea et al. noted cerebral vasculopathy in 17 of 266 children (6%, 95% CI: 4–10%) with neurofibromatosis type 1, an 8% difference in proportions, which also was significant (95% CI: 4–16%, p=0.02).
DISCUSSION
Vasculopathy is an increasingly-recognized complication of neurofibromatosis type 1. However, the spectrum of vascular abnormalities that may present in pediatric neurofibromatosis type 1 has not been well-characterized, particularly for peripheral lesions. We report a minimum prevalence rate of 8% for both peripheral and cerebral vasculopathy associated with pediatric neurofibromatosis type 1, based on confirmed findings in 14 of 181 children seen in a large neurofibromatosis clinic. At our center, the minimum prevalence of symptomatic peripheral vascular abnormalities was 2 of 181 children (1%) with neurofibromatosis type 1. The minimum prevalence of symptomatic cerebrovascular disease was 2 of 181 children (1%). When children with asymptomatic as well as symptomatic cerebrovascular disease were included, the minimum prevalence was 12 of 181 children (7%) with a prevalence of 15% in 77 children with appropriate neuroimaging. The actual prevalence of vasculopathy may be greater, as only 80 children (44%) of the clinic population underwent imaging studies, and even fewer had dedicated vascular imaging of the brain (11/181, 6%) or peripheral vasculature (6/181, 3%). Cerebral vasculopathy was assessed primarily by abnormal flow voids on magnetic resonance imaging; our study is similar to prior reports of cerebral vasculopathy in this regard. In Rosser et al. 8/316 children (2.5%) had magnetic resonance angiography of the brain, in Rea et al. 35/266 children (13%) had magnetic resonance angiography available to assess for vasculopathy, and Cairns & North had 7/144 children with magnetic resonance angiography (5%).4–6
Cerebral vasculopathy was often asymptomatic – of the patients with vascular abnormalities identified on neuro-radiological studies, only 2/12 (17%) presented with classic stroke-like symptoms. The remaining patients were discovered incidentally on scans performed for other reasons (Table 1), for example magnetic resonance imaging of the brain was done for headache (one patient) or with concerns for optic pathway glioma (four patients). Four children (Table 3, #7, 9, 11, and 12) were found to have previously unidentified vascular abnormalities (all asymptomatic vessel tortuosity or elongation). In children imaged for clinical indications, 12 of 77 (15%) with magnetic resonance imaging of the brain had cerebral vasculopathy, which is a higher frequency than has been reported in other neurofibromatosis clinic populations (2–6%) but is still within the 95% confidence intervals of 2 of 3 prior publications.4–6 Review of all scans by neuroradiologists (rather than relying on previous radiology reports) may have increased this study’s sensitivity for mild vascular anomalies, contributing to our higher prevalence of vasculopathy.
Moyamoya syndrome was the most common vascular finding in this study, and has been shown to be a common vasculopathy seen in neurofibromatosis type 1.6 Of the five patients with moyamoya, three had no symptoms of stroke or transient ischemic attack at the time of diagnosis (though one had silent stroke on brain magnetic resonance imaging done for optic pathway glioma monitoring), while two had symptomatic cerebral ischemia (stroke or transient ischemic attack).
Peripheral vasculopathy is more elusive. Only children with significantly elevated blood pressures were regularly evaluated via imaging. Children with milder blood pressure elevation were often not imaged, so the current results represent a minimum prevalence of peripheral vasculopathy in children with neurofibromatosis type 1. Two patients (#13, 14) were found to have mid-aortic syndrome, which was the only peripheral lesion identified in our study population. Mid-aortic syndrome refers to aortic coarctation at the level of the distal thoracic or abdominal aorta, and has been reported in association with neurofibromatosis type 1.14,21,22 Mid-aortic syndrome is frequently accompanied by renal and visceral artery stenosis, and most patients present with uncontrollable hypertension (as was the case with the two affected children in this study).23 Lin et al. reviewed over 2500 adult and pediatric patients in the National Neurofibromatosis Foundation International Database for cardiovascular malformations, including aortic coarctation, and found an overall frequency of 2%.24 To our knowledge, ours is the first study to consider all types of cerebral and peripheral vascular abnormalities in children with neurofibromatosis type 1. In this small sample, we found that symptomatic peripheral vascular problems (n=2) were not associated with cerebrovascular problems.
Cerebral vasculopathy was not strongly associated with optic pathway glioma in children with neurofibromatosis type 1, contrary to previous studies. One explanation for this lack of association is simply that our sample size is small. Another possibility is that no child in this study was treated with cranial radiation for optic pathway glioma. Radiation is a known cause of cerebral vasculopathy.25 Finally, patients with optic pathway glioma are more likely to have serial imaging to monitor for optic pathway glioma progression and therefore a progressive cerebral vasculopathy may be identified when there is not a true statistical association. Indeed, three cases of moyamoya in this study were discovered incidentally during routine scans for optic pathway glioma monitoring.5
There are several limitations to this study. A selection bias is inherent to the recruitment of patients from a tertiary care clinic. Our study population may have included a greater number of patients with more advanced or difficult-to-manage disease and therefore more children with vasculopathy; however, overall we seemed to have a fairly typical population of children with neurofibromatosis type 1 in that 49% of cases were familial, 29% had attention deficit hyperactivity disorder, 53% had suspected learning disability and 36% had plexiform neurofibroma.26,27 As a neurology-based NF clinic, we had more children with glioma, particularly optic pathway glioma and more cerebral vasculopathy than has been reported in studies done in genetics-based clinics.17 However, we would not expect to have a higher than typical prevalence of peripheral vascular disease or hypertension. Lastly, not all patients with identified vascular abnormalities were evaluated via conventional angiography. Magnetic resonance angiography is limited by spatial resolution and over-estimation of stenosis.28 In our study, most vasculopathy was noted on magnetic resonance imaging and blood vessel imaging was simply confirmatory.
From this work, we can conclude that significant hypertension in a child with neurofibromatosis type 1 may indicate a renal or aortic vasculopathy. Further, from this relatively small dataset, it is apparent that there is not a strong association between peripheral vascular and cerebrovascular abnormalities in neurofibromatosis type 1; this study is underpowered to detect lesser associations.
There is an existing recommendation from one group of researchers that all children with neurofibromatosis type 1 who are undergoing neuroimaging should have a cerebral magnetic resonance angiography included in their studies because neurofibromatosis type 1 arteriopathy may be asymptomatic and potentially progressive.5 We would add that while hypertension in children with neurofibromatosis type 1 is uncommon (6% of our study population), those with significant hypertension should be screened for renal and aortic vasculopathy. In our small sample, 40% of children with hypertension had a vascular cause. Awareness of vascular pathology in pediatric patients with neurofibromatosis type 1 is important not only for standard clinical care, but it is a factor that should be considered when developing clinical trials and therapeutics for patients with neurofibromatosis type 1. For example, patients with known vascular disease will need special review when considering enrollment in clinical trials for their other neurofibromatosis type 1 manifestations with agents that may exacerbate vaso-occlusive disease (i.e. anti-angiogenesis agents). Conversely, some drugs currently in trial for plexiform neurofibroma (i.e. imatinib) or cognitive processing (i.e. lovastatin) may in fact positively impact vascular disease.10,29
Acknowledgements
Work was performed at the Departments of Neurology and Radiology and Radiological Science at the Johns Hopkins Hospital, Baltimore, Maryland.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article. Dr. Jordan is supported by NIH K23 - NS062110 to study stroke in children.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Bonnie Kaas, Email: bkaas1@jhmi.edu, Johns Hopkins University School of Medicine.
Thierry A.G.M. Huisman, Email: thuisma1@jhmi.edu, Johns Hopkins Dept of Radiology and Radiological Science.
Aylin Tekes, Email: atekes1@jhmi.edu, Johns Hopkins Dept of Radiology and Radiological Science.
Amanda Bergner, Email: abergne1@jhmi.edu, Johns Hopkins Dept of Neurology.
Jaishri O. Blakeley, Email: jblakel3@jhmi.edu, Johns Hopkins Dept of Neurology.
Lori C. Jordan, Johns Hopkins Dept of Neurology, now Vanderbilt University Dept of Neurology
References
- 1.Cawthon RM, Weiss R, Xu GF, et al. A major segment of the neurofibromatosis type 1 gene: CDNA sequence, genomic structure, and point mutations. Cell. 1990;62:193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- 2.Xu GF, O'Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Ismat FA, Wang T, et al. NF1 regulates a ras-dependent vascular smooth muscle proliferative injury response. Circulation. 2007;116:2148–2156. doi: 10.1161/CIRCULATIONAHA.107.707752. [DOI] [PubMed] [Google Scholar]
- 4.Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology. 2005;64:553–555. doi: 10.1212/01.WNL.0000150544.00016.69. [DOI] [PubMed] [Google Scholar]
- 5.Rea D, Brandsema JF, Armstrong D, et al. Cerebral arteriopathy in children with neurofibromatosis type 1. Pediatrics. 2009;3:e476–e483. doi: 10.1542/peds.2009-0152. [DOI] [PubMed] [Google Scholar]
- 6.Cairns AG, North KN. Cerebrovascular dysplasia in neurofibromatosis type 1. J Neurol Neurosurg Psychiatry. 2008;79:1165–1170. doi: 10.1136/jnnp.2007.136457. [DOI] [PubMed] [Google Scholar]
- 7.Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: Report of the NF1 cardiovascular task force. Genet Med. 2002;4:105–111. doi: 10.1097/00125817-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Norton KK, Xu J, Gutmann DH. Expression of the neurofibromatosis I gene product, neurofibromin, in blood vessel endothelial cells and smooth muscle. Neurobiol Dis. 1995;2:13–21. doi: 10.1006/nbdi.1995.0002. [DOI] [PubMed] [Google Scholar]
- 9.Ahlgren-Beckendorf JA, Maggio WW, Chen F, Kent TA. Neurofibromatosis 1 mRNA expression in blood vessels. Biochem Biophys Res Commun. 1993;197:1019–1024. doi: 10.1006/bbrc.1993.2580. [DOI] [PubMed] [Google Scholar]
- 10.Lasater EA, Bessler WK, Mead LE, et al. Nf1 +/− mice have increased neointima formation via hyperactivation of a Gleevec sensitive molecular pathway. Hum Mol Genet. 2008;17:2336–2344. doi: 10.1093/hmg/ddn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton SJ, Friedman JM. Insights into the pathogenesis of neurofibromatosis 1 vasculopathy. Clin Genet. 2000;58:341–344. doi: 10.1034/j.1399-0004.2000.580501.x. [DOI] [PubMed] [Google Scholar]
- 12.Kurien A, John PR, Milford DV. Hypertension secondary to progressive vascular neurofibromatosis. Arch Dis Child. 1997;76:454–455. doi: 10.1136/adc.76.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hersh JH. American Academy of Pediatrics Committee on Genetics. Health supervision for children with neurofibromatosis. Pediatrics. 2008;121:633–642. doi: 10.1542/peds.2007-3364. [DOI] [PubMed] [Google Scholar]
- 14.Criado E, Izquierdo L, Lujan S, et al. Abdominal aortic coarctation, renovascular, hypertension, and neurofibromatosis. Ann Vasc Surg. 2002;16:363–367. doi: 10.1007/s10016-001-0098-4. [DOI] [PubMed] [Google Scholar]
- 15.Oderich GS, Sullivan TM, Bower TC, et al. Vascular abnormalities in patients with neurofibromatosis syndrome type I: Clinical spectrum, management, and results. J Vasc Surg. 2007;46:475–484. doi: 10.1016/j.jvs.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 16.Han M, Criado E. Renal artery stenosis and aneurysms associated with neurofibromatosis. J Vasc Surg. 2005;41:539–543. doi: 10.1016/j.jvs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: Consensus statement from the NF1 optic pathway glioma task force. Ann Neurol. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 18.Adolescent Idiopathic Scoliosis: Treatment; [about 3 screens] Milwaukee, WI: c2011. [cited 2010 Jun 18]. Scoliosis Research Society [homepage on the Internet] Available from: http://www.srs.org/professionals/conditions_and_treatment/adolescent_idiopathic_scoliosis/treatment.htm. [Google Scholar]
- 19.Weinstein SL, Dolan LA, Cheng JC, et al. Adolescent idiopathic scoliosis. The Lancet. 2008;371:1527–1537. doi: 10.1016/S0140-6736(08)60658-3. [DOI] [PubMed] [Google Scholar]
- 20.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 21.West CA, Delis KT, Service GJ, et al. Middle aortic syndrome: Surgical treatment in a child with neurofibromatosis. J Vasc Surg. 2005;42:1236. doi: 10.1016/j.jvs.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Connolly JE, Wilson SE, Lawrence PL, Fujitani RM. Middle aortic syndrome: Distal thoracic and abdominal coarctation, a disorder with multiple etiologies. J Am Coll Surg. 2002;194:774–781. doi: 10.1016/s1072-7515(02)01144-4. [DOI] [PubMed] [Google Scholar]
- 23.Stanley JC, Criado E, Eliason JL, et al. Abdominal aortic coarctation: Surgical treatment of 53 patients with a thoracoabdominal bypass, patch aortoplasty, or interposition aortoaortic graft. J Vasc Surg. 2008;48:1073–1082. doi: 10.1016/j.jvs.2008.05.078. [DOI] [PubMed] [Google Scholar]
- 24.Lin AE, Birch PH, Korf BR, et al. Cardiovascular malformations and other cardiovascular abnormalities in neurofibromatosis 1. Am J Med Genet. 2000;95:108–117. doi: 10.1002/1096-8628(20001113)95:2<108::aid-ajmg4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45:393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12:1–11. doi: 10.1097/GIM.0b013e3181bf15e3. [DOI] [PubMed] [Google Scholar]
- 27.Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: A twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 28.Ruggieri P, Masaryk T, Ross J, Modic M. Magnetic resonance angiography of the intracranial vasculature. Top Magn Reson Imaging. 1991;3:23–33. [PubMed] [Google Scholar]
- 29.Acosta MT, Kardel PG, Walsh KS, et al. Lovastatin as treatment for neurocognitive deficits in neurofibromatosis type 1: phase I study. Pediatr Neurol. 2010;4:241–245. doi: 10.1016/j.pediatrneurol.2011.06.016. [DOI] [PubMed] [Google Scholar]