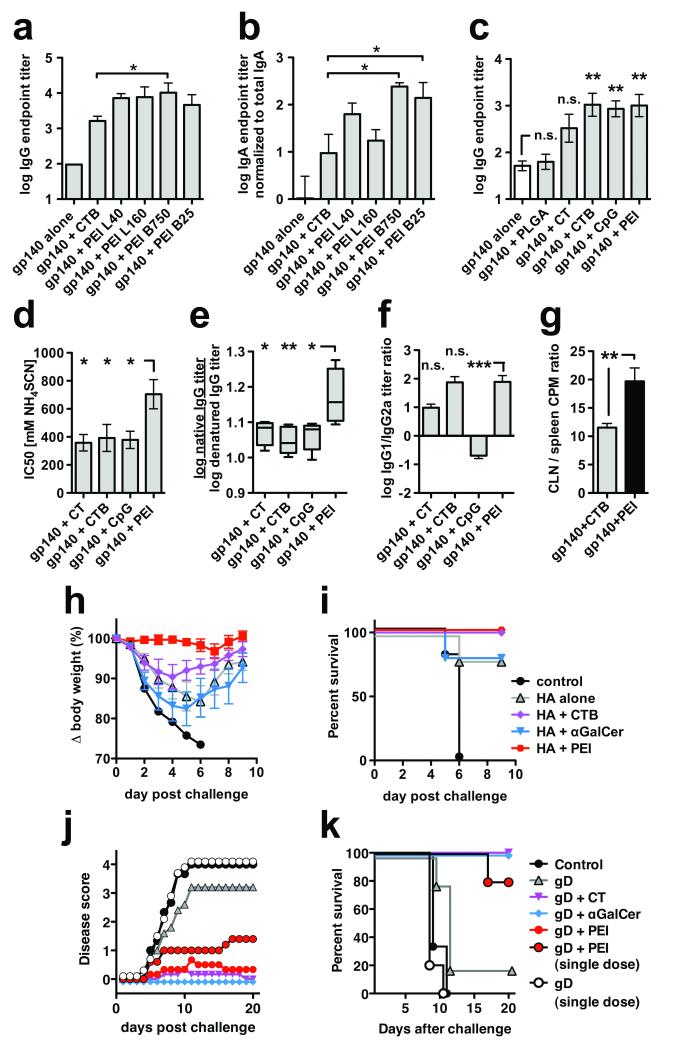
Figure 1.
Mucosal adjuvant activity of PEI and protection from disease by mucosal pathogens. (a,b) BALB/c mice (n=6) were primed and boosted once intranasally with 10 μg HIV-1 gp140 combined with 10 μg CTB or 10 μg of linear (L) or branched (B) PEI at different molecular weights (kDa) as indicated; (a) gp140-specific serum IgG and (b) vaginal IgA four weeks post-boost immunization (Anova/Bonferroni test). (c-f) BALB/c mice (n=5) were immunized intranasally with two doses of 10 μg HIV-1 gp140 combined with 20 μg CTB, CpG, PEI, 2 μg CT or 1 mg PLGA nanoparticles. (c) gp140-specific serum IgG on day 21 post-prime immunization (Anova/Bonferroni test). (d) gp140-specific serum IgG avidity index (2 weeks post-boost) measured by chaotropic ELISA using NH4SCN (Anova/Dunnett); high avidity interactions can tolerate higher chaotropic agent concentrations than low avidity interactions. (e) ratio of serum IgG endpoint titers against native versus SDS/DTT-denatured gp140 (2 weeks post-boost; Anova/Bonferroni). (f) Serum IgG1 / IgG2a titer ratio (2 weeks post-boost; Kruskal-Wallis/Dunn), data representative of 2 experiments. (g) BALB/c mice (n=6) were immunized intranasally with three doses of gp140 alone or adjuvanted with CTB or PEI and the ratio of local (CLN, cervical lymph node) proliferation versus systemic (spleen) proliferation was determined by 3H-thymidine incorporation (unpaired t-test). (h,i) Protection of mice from highly pathogenic influenza virus challenge, data shown are representative of three independent experiments. C57Bl/6 mice (n=4-5) were immunized intranasally with a single dose of 10 μg influenza A (PR8) HA alone or combined with 2 μg αGalCer, 20 μg CTB or 20 μg PEI, followed by intranasal challenge three weeks later with a highly pathogenic dose of live influenza A PR8 virus. (h) Weight loss post-challenge. (i) Kaplan-Meier survival plots. (j,k) Protection of mice from highly pathogenic vaginal HSV-2 challenge. C57Bl/6 mice (n=7) were immunized intranasally with one or three doses of 5 μg HSV-2 gD combined with 20 μg CpG, PEI or 2 μg αGalCer per administration, followed by vaginal challenge (n=5) with previously titrated HSV-2. (j) Disease score post-HSV-2 challenge (k) Kaplan-Meier survival plots. All data are presented as mean of replicates from individual experiments ± SEM; n.s., not significant; *p<0.05, **p<0.01, ***p<0.001