Abstract
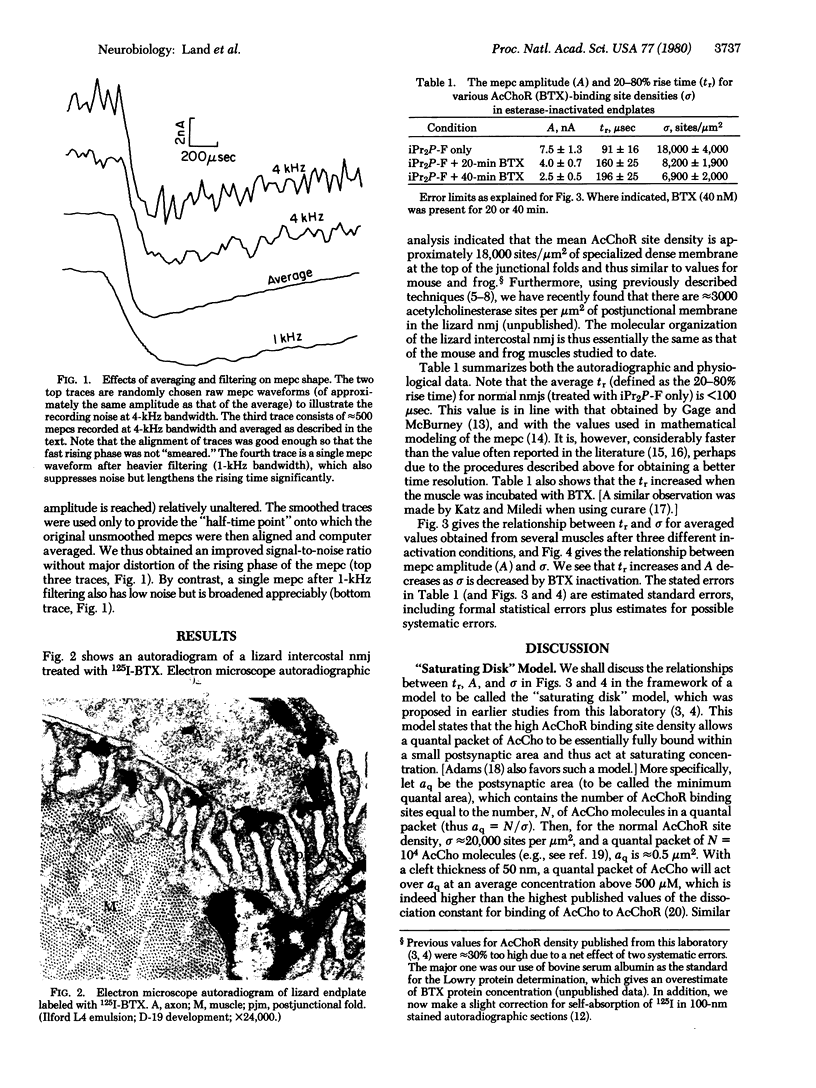
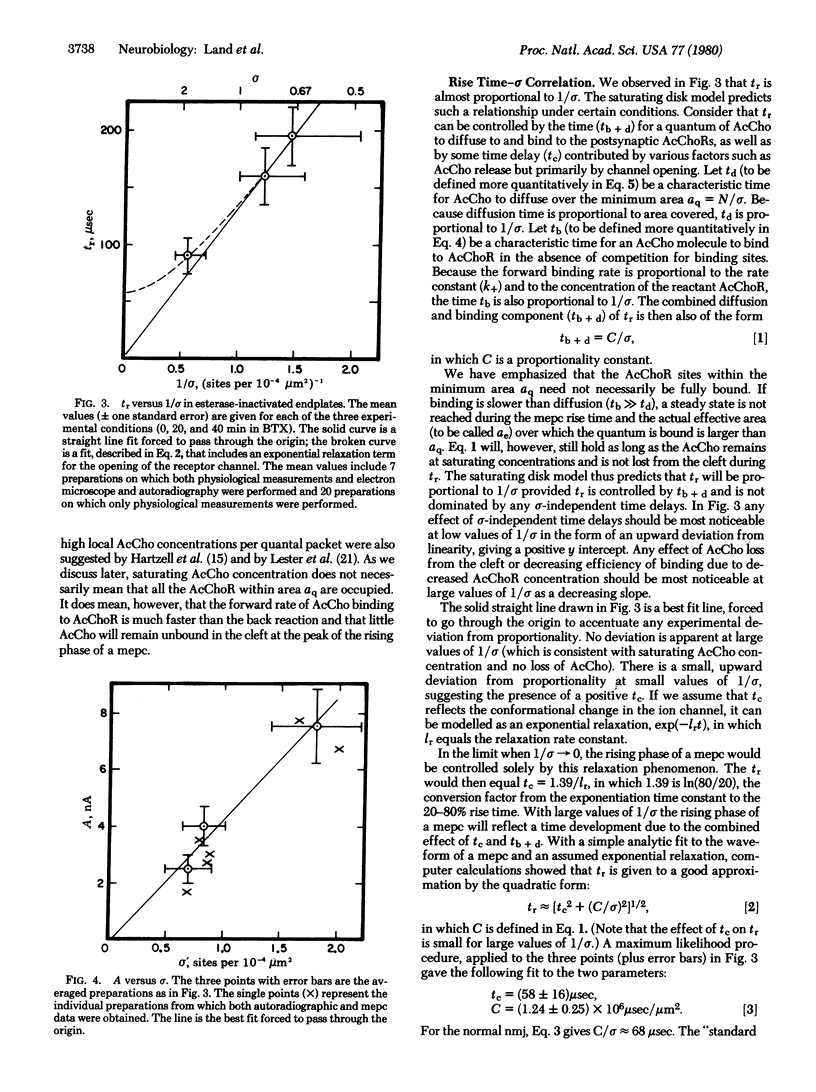
The relationship between acetylcholine receptor (AcChoR) site density (sigma) and the rising phase of the miniature endplate current was determined in esterase-inactivated lizard intercostal neuromuscular junctions. The currents were recorded by using a voltage clamp. The receptor site density was determined by electron microscope autoradiography after labeling with 125I-labeled alpha-bungarotoxin in normal endplates and in those partially inactivated with nonradioactive alpha-bungarotoxin. We found that as sigma is decreased the rise time in increased and the amplitude is decreased. These results are compatible with a previously stated "saturating disk" model, which suggests that a quantum of acetylcholine (AcCho) acts on a small postsynaptic area at saturating concentration. We conclude that in the normal neuromuscular junction the most likely number of AcCho molecules needed to open an ion channel is 2, and that the 20--80% rise time of < 100 musec is influenced both by the sigma-dependent factors such as diffusion and binding of AcCho to AcChoR and by the sigma-independent time delays such as the conformation change time to open the ion channels. From our data we calculate the lower limits to the forward rate constant of AcCho binding to AcChoR greater than or equal to 3 X 10(7) M-1 sec-1 and the diffusion constant for AcCho in the cleft greater than or equal to 4 X 10(-6) cm2 sec-1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. End-plate currents and acetylcholine noise at normal and myasthenic human end-plates. J Physiol. 1979 Feb;287:247–265. doi: 10.1113/jphysiol.1979.sp012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Sensitivity in electron microscope autoradiography for 125I. J Histochem Cytochem. 1974 Feb;22(2):80–87. doi: 10.1177/22.2.80. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Salpeter S. R. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol. 1979 Jul;82(1):150–173. doi: 10.1083/jcb.82.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. Diffusion of acetylcholine in agar gels and in the isolated rat diaphragm. J Physiol. 1960 Oct;153:562–572. doi: 10.1113/jphysiol.1960.sp006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Chang S. L., Kau S. T., Luh S. H. Chromatographic separation of the venom of Bungarus multicinctus and characterization of its components. J Chromatogr. 1972 Oct 5;72(1):71–82. doi: 10.1016/0021-9673(72)80009-8. [DOI] [PubMed] [Google Scholar]
- Lester H. A., Koblin D. D., Sheridan R. E. Role of voltage-sensitive receptors in nicotinic transmission. Biophys J. 1978 Mar;21(3):181–194. doi: 10.1016/S0006-3495(78)85518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Cohen J. B. Equilibrium binding of [3H]tubocurarine and [3H]acetylcholine by Torpedo postsynaptic membranes: stoichiometry and ligand interactions. Biochemistry. 1979 Nov 27;18(24):5464–5475. doi: 10.1021/bi00591a032. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Barnard E. A., Chiu T. H. The ultrastructural localization and quantitation of cholinergic receptors at the mouse motor endplate. J Membr Biol. 1973;14(4):383–402. doi: 10.1007/BF01868086. [DOI] [PubMed] [Google Scholar]
- Rogers A. W., Darzynkiewicz Z., Salpeter M. M., Ostrowski K., Barnard E. A. Quantitative studies on enzymes in structures in striated muscles by labeled inhibitor methods. I. The number of acetylcholinesterase molecules and of other DFP-reactive sites at motor endplates, measured by radioautography. J Cell Biol. 1969 Jun;41(3):665–685. doi: 10.1083/jcb.41.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L. Quantitative simulation of endplate currents at neuromuscular junctions based on the reaction of acetylcholine with acetylcholine receptor and acetylcholinesterase. Biophys J. 1979 May;26(2):263–289. doi: 10.1016/S0006-3495(79)85249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALPETER M. M., BACHMANN L. AUTORADIOGRAPHY WITH THE ELECTRON MICROSCOPE. A PROCEDURE FOR IMPROVING RESOLUTION, SENSITIVITY, AND CONTRAST. J Cell Biol. 1964 Aug;22:469–477. doi: 10.1083/jcb.22.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M. Electron microscope radioautography as a quantitative tool in enzyme cytochemistry. II. The distribution of DFP-reactive sties at motor endplates of a vertebrate twitch muscle. J Cell Biol. 1969 Jul;42(1):122–134. doi: 10.1083/jcb.42.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Plattner H., Rogers A. W. Quantitative assay of esterases in end plates of mouse diaphragm by electron microscope autoradiography. J Histochem Cytochem. 1972 Dec;20(12):1059–1068. doi: 10.1177/20.12.1059. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Rogers A. W., Kasprzak H., McHenry F. A. Acetylcholinesterase in the fast extraocular muscle of the mouse by light and electron microscope autoradiography. J Cell Biol. 1978 Jul;78(1):274–285. doi: 10.1083/jcb.78.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]



