Figure 1.
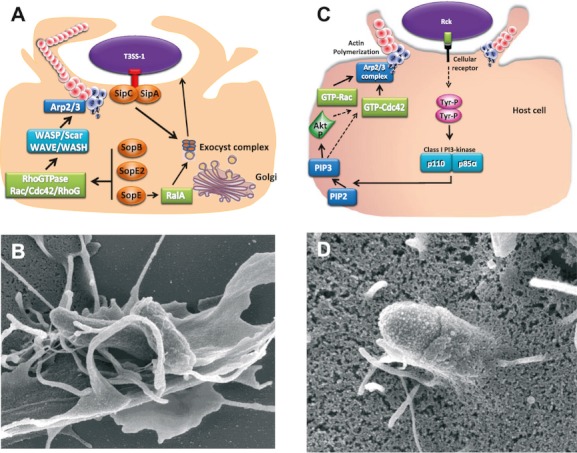
Trigger and Zipper mechanisms used by Salmonella to enter cells. (A) Schematic representation of the Trigger mechanism: Using a type III secretion system (T3SS), Salmonella bacterial effectors (SipA, SipC, SopB, SopE, SopE2) are directly injected into eukaryotic cells. SopE, SopE2, and SopB activate the RhoGTPases Rac/Cdc42/RhoG to allow actin cytoskeleton remodeling via cellular proteins, such as WASP/Scar/WAVE/WASH, which activate the Arp2/3 complex. In contrast, SipA and SipC bind directly to actin. To induce the formation of membrane ruffles and internalization, the recruitment of the exocyst complex is required and is manipulated by SipC and by SopE via the Ras-related protein RalA. (B) Scanning electron microscopy of Salmonella entering into cells via a Trigger mechanism, which is characterized by the apparition of large membrane ruffles at the bacterial entry site. (C) Schematic representation of the Zipper mechanism: the Rck invasin expressed on Salmonella outer membrane interacts with its receptor on the host cell membrane, leading to phosphorylation of at least one tyrosine kinase. Activation of the class I PI 3-kinase induces PI (3,4,5)P3 formation, participating to Akt activation. The activation of the GTPase Rac1, downstream of the Akt/PI 3-kinase activation, and the GTPase Cdc42 trigger actin polymerization via the Arp2/3 nucleator complex. The mechanism controlling Cdc42 during Rck-induced signaling pathway is still unknown. Dotted arrows represent possible signaling events and/or interactions. (D) Scanning electron microscopy of Salmonella entering into cells via a Zipper mechanism, which is characterized by weak membrane rearrangements.
