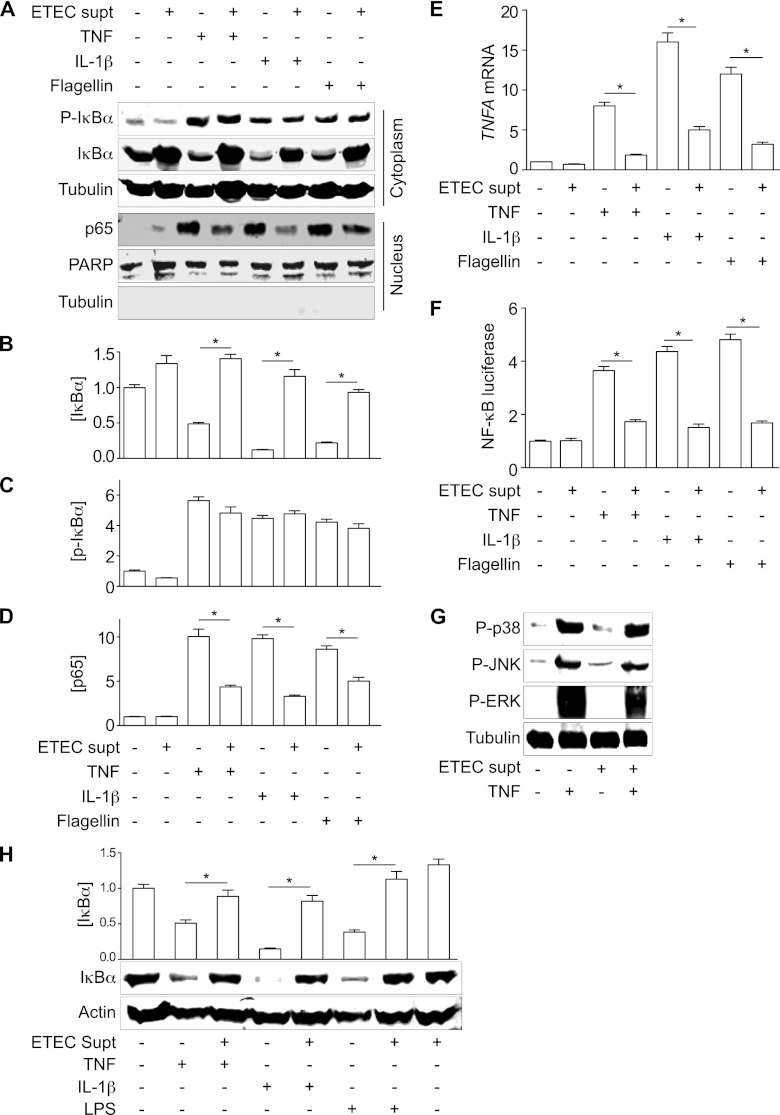
Fig 3.
ESF inhibits NF-κB nuclear translocation and transcriptional activity. (A) Immunoblotting of total and phospho(Ser32/36)-IκBα, as well as nucleus-localized NF-κB p65, after pretreating HCT-8 cells with ETEC supernatant for 1.5 h and then stimulating the cells with either TNF (20 ng/ml), IL-1β (10 ng/ml), or flagellin (200 ng/ml) for 30 min. PARP and tubulin immunoblotting were used to normalize nuclear protein concentrations and to demonstrate the absence of contaminating cytoplasmic proteins in the nuclear fractions, respectively. (B to D) Quantification of IκBα, phospho(Ser32/36)-IκBα, and p65 abundance from data shown in panel A. Asterisks indicate significantly different protein abundance between the specified pairwise comparisons. (E) RT-PCR analysis of TNF-α gene (TNFA) transcription after treating cells with TNF, IL-1β, or flagellin, with or without the ETEC supernatant. Asterisks indicate significantly different protein abundance between the specified pairwise comparisons. (F) NF-κB luciferase activity after treating cells with TNF, IL-1β, or flagellin, with or without the ETEC supernatant. Asterisks indicate significantly different protein abundance between the specified pairwise comparisons. (G) Immunoblotting of phospho-p38, phospho-JNK, and phospho-ERK1/2 after ETEC supernatant and TNF treatment. (H) IκBα immunoblotting after treating differentiated THP-1 cells with TNF (20 ng/ml), IL-1β (10 ng/ml), or LPS (1 μg/ml) for 30 min, with or without the ETEC supernatant. Asterisks indicate significantly different protein abundance between the specified pairwise comparisons.