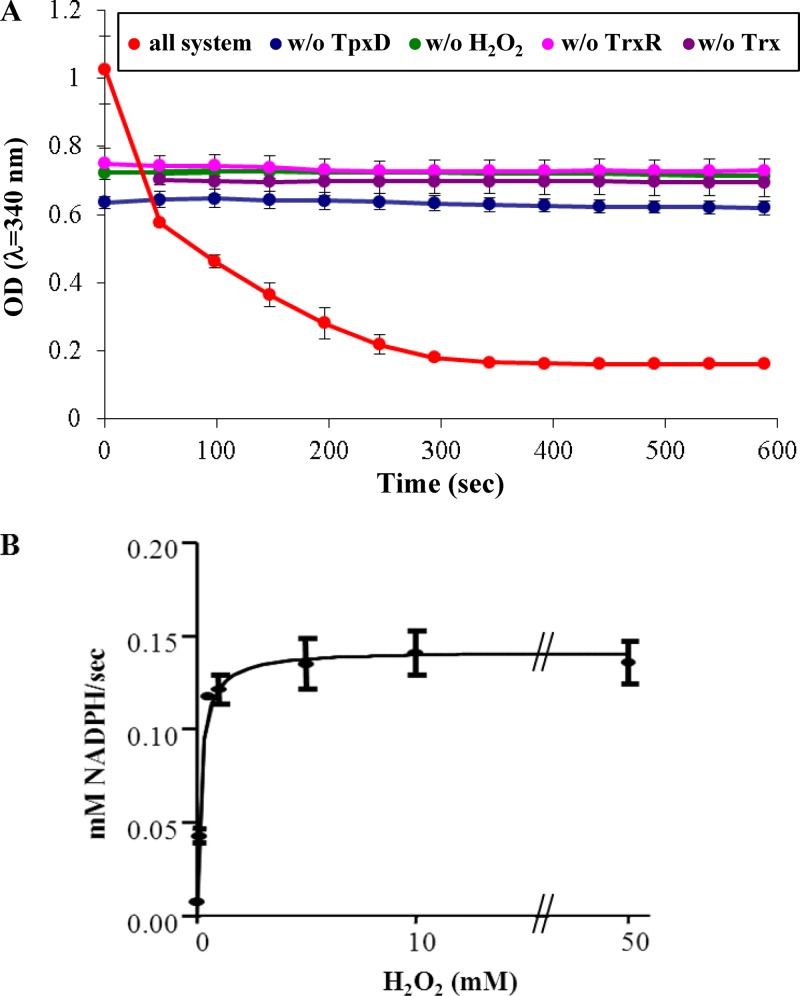
Fig 1.
Peroxidase activity of recombinant TpxD using the thioredoxin system as a reductant. The reaction mixture contained 100 mM HEPES-NaOH (pH 7.0), 0.2 mM NADPH, 3.4 mM E. coli Trx, 0.36 mM E. coli TrxR, 5 μM purified TpxD, and 1 mM H2O2. Reactions were carried out with a total volume of 0.2 ml at 25°C and were started by the addition of H2O2 to the mixture. (A) Each curve is an absorbance time course at 340 nm, due to NADPH oxidation. In control experiments, TpxD (blue), H2O2 (green), thioredoxin reductase (pink), or thioredoxin (violet) was omitted from the reaction mixture. The data shown are from one representative experiment done in duplicates, but it was repeated 3 times, with similar results. (B) Michaelis-Menten curve for the determination of Km, Vmax, and Kcat/Km values, with H2O2 concentrations plotted on the x axis and velocity plotted on the y axis.