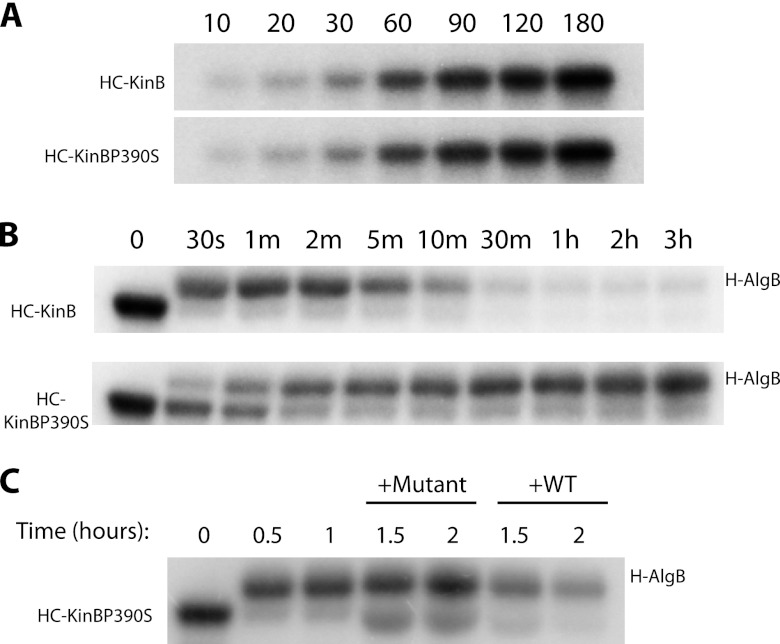
Fig 6.
KinB acts as a phosphatase to regulate AlgB activity. Autophosphorylation and phosphotransfer assays were performed in vitro with radiolabeled ATP and labeled proteins were separate by SDS-PAGE. (A) Autophosphorylation of HC-KinB and HC-KinBP390S. (B) Kinetics of phosphotransfer of the radiolabel from HC-KinB to H-AlgB (top) and from HC-KinBP390S to H-AlgB (bottom). (C) Autophosphorylated HC-KinBP390S (lane 1) was incubated with H-AlgB, resulting in phosphotransfer within 30 min to 1 h (lanes 2 and 3). Subsequent addition of unlabeled WT (HC-KinB; lanes 6 and 7) but not unlabeled mutant (HC-KinBP390S; lanes 4 and 5) KinB protein to phosphotransfer reactions resulted in loss of the radiolabel from H-AlgB. All results are representative of three replicates.