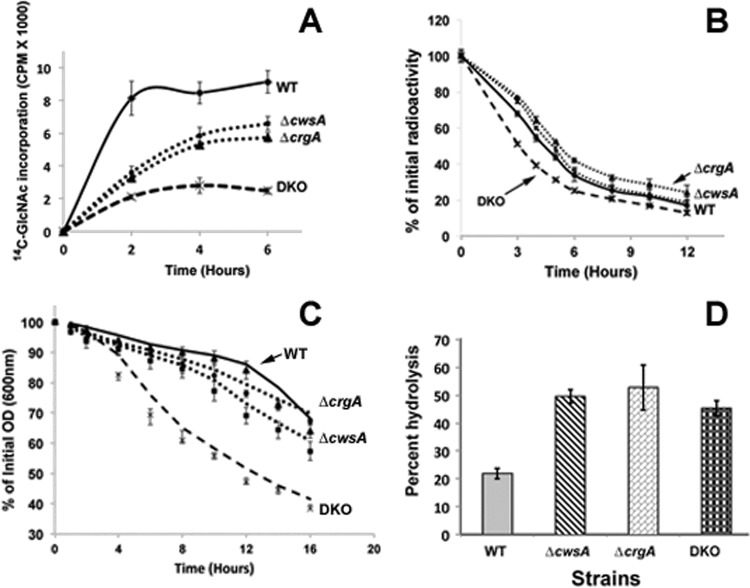
Fig 8.
Loss of crgA and cwsA causes severe defects in cell wall synthesis. (A) 14C-labeled GlcNAc incorporation in M. smegmatis WT, ΔcwsA, ΔcrgA, and DKO strains. Exponential cultures were grown with 14C-labeled GlcNAc as described in Materials and Methods. At 1 min, 2 h, 4 h, and 6 h after addition of the label, 0.5 ml of culture was filtered through a 0.2-μm-pore-size nitrocellulose filter and washed, the filters were air dried, and radioactivity was measured in a liquid scintillation counter. Data shown are normalized to the A600 at each given point. Means ± standard deviations from 3 independent experiments are shown. (B) Cell wall turnover in M. smegmatis strains from panel A. Cells were labeled with [14C]GlcNAc for 6 h as described for panel A and pelleted. The labeled cells were resuspended in medium without label and grown at 37°C with shaking. At 0, 3, 4, 5, 6, 8, 10, and 12 h following growth in label-free medium, 0.5 ml of culture was pelleted, boiled in 4.0% SDS for 30 min, and passed through a 0.22-μm-pore-size filter. The filters were processed as described in Materials and Methods, and the retained radioactivity was measured in a liquid scintillation counter. Percent PG turnover at each time point was calculated with respect to that at time zero. Marker labels are as described for panel A. Means ± standard deviations from 3 independent experiments are shown. Statistical analysis of data using paired Student's t test indicated significant differences between WT and the ΔcrgA strain (P = 0.003), WT and DKO (P = 0.005), and WT and the ΔcwsA strain (P = 0.011). One-way ANOVA followed by Tukey's multiple-comparison test revealed significant differences between the ΔcrgA and ΔcwsA strains (P = 0.0035), the ΔcrgA and DKO strains (P = 0.0058), and the ΔcwsA and DKO strains (P = 0.0249). (C) Autolysis of M. smegmatis strains. Various M. smegmatis strains (as described for panel A) were grown to an A600 of 0.5, and cells were pelleted, resuspended in 50 mM glycine, pH 8.0, containing 0.05% Tween 20, and incubated with shaking at 37°C. At time points of 0, 1, 2, 4, 6, 8, 10, 12, 14, and 16 h, aliquots of cells were removed and the A600 was measured. The percentage of the initial optical density (OD) retained was calculated with respect to that at time zero. Data shown are means ± standard deviations from 3 independent experiments. Paired Student's t test showed significant differences in autolysis between the WT and ΔcwsA strains (P = 0.0078), the WT and DKO strains (P = 0.0028), the ΔcwsA and DKO strains (P = 0.0026), and the ΔcrgA and DKO strains (P = 0.0014) but not between the WT and the ΔcwsA strains (P = 0.063). (D) Lysozyme sensitivity. Fluorescein-labeled cell walls were prepared from WT and mutant M. smegmatis strains as described in the text and incubated with 1 mg/ml lysozyme in a buffer containing 50 mM Tris and 1 mM EDTA for 4 h. Reactions were stopped by addition of an equal volume of 4 M LiCl and spun, and the fluorescent label released in the supernatant was quantitated in a Jasco FP6500 fluorimeter. Reactions were done in triplicate, and average percent hydrolysis is shown.