Figure 1. Albumin transport across the alveolo-capillary barrier.
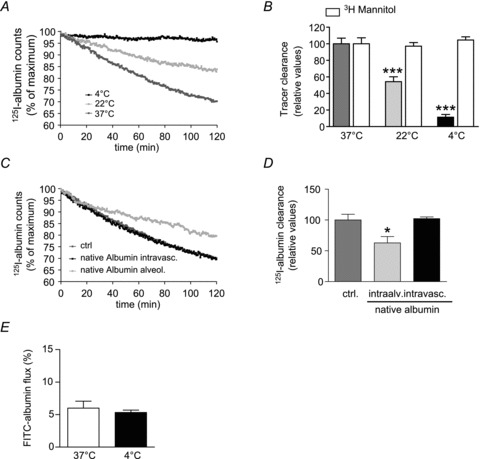
A, isolated, ventilated and perfused rabbit lungs were maintained either at 37°C (dark grey), 22°C (light grey) or 4°C (black). 125I-albumin was deposited into the alveolar space by nebulization. Counts were set at 100% immediately after nebulization. Each data point represents the mean of at least six independent experiments. For clarity, standard deviations have been omitted; however, they are incorporated into analyses of these data in B. B, 125I-albumin transport was quantified from the data presented in A. The [3H]mannitol flux (open bars) in lungs maintained at 37°C was set at 100%, while mannitol flux in lungs maintained at low temperature was expressed relative to this control value. Data represent the mean ± SD (n = 6 for all groups). C, control lungs (dark grey) were maintained at 37°C and sham-nebulized with normal saline after establishing steady-state equilibrium. A 1000-fold molar excess of native albumin was nebulized into the lungs (light grey). Alternatively, excess native albumin (20 mg ml−1) was applied to the perfusate of the lungs (black). 125I-albumin tracer was nebulized to the lungs, and elimination of this tracer from the lung was monitored. D, active 125I-albumin transport was set at 100% in control lungs and active transport of the tracer in excess native albumin-treated lungs was expressed relative to this control value. E, FITC-albumin was administered to the perfusate and quantified in the ELF from BAL samples. The FITC-albumin concentration in the perfusate was set to 100%, and the concentration of FITC in the BAL was calculated as a percentage of the FITC concentration in the perfusate. Bars represent the mean ± SEM (n = 6 for all groups); *P < 0.05, ***P < 0.001.
